Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery - PubMed (original) (raw)
Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery
Greg A Knock et al. Cardiovasc Res. 2008.
Abstract
Aims: We investigated the role of src family kinases (srcFK) in agonist-mediated Ca2+-sensitization in pulmonary artery and whether this involves interaction with the rho/rho-kinase pathway.
Methods and results: Intra-pulmonary arteries (IPAs) and cultured pulmonary artery smooth muscle cells (PASMC) were obtained from rat. Expression of srcFK was determined at the mRNA and protein levels. Ca2+-sensitization was induced by prostaglandin F(2 alpha) (PGF(2 alpha)) in alpha-toxin-permeabilized IPAs. Phosphorylation of the regulatory subunit of myosin phosphatase (MYPT-1) and of myosin light-chain-20 (MLC20) and translocation of rho-kinase in response to PGF(2 alpha) were also determined. Nine srcFK were expressed at the mRNA level, including src, fyn, and yes, and PGF(2 alpha) enhanced phosphorylation of three srcFK proteins at tyr-416. In alpha-toxin-permeabilized IPAs, PGF(2 alpha) enhanced the Ca2+-induced contraction (pCa 6.9) approximately three-fold. This enhancement was inhibited by the srcFK blockers SU6656 and PP2 and by the rho-kinase inhibitor Y27632. Y27632, but not SU6656 or PP2, also inhibited the underlying pCa 6.9 contraction. PGF(2 alpha) enhanced phosphorylation of MYPT-1 at thr-697 and thr-855 and of MLC20 at ser-19. This enhancement, but not the underlying basal phosphorylation, was inhibited by SU6656. Y27632 suppressed both basal and PGF(2 alpha)-mediated phosphorylation. The effects of SU6656 and Y27632, on both contraction and MYPT-1 and MLC20 phosphorylation, were not additive. PGF(2 alpha) triggered translocation of rho-kinase in PASMC, and this was inhibited by SU6656.
Conclusions: srcFK are activated by PGF(2 alpha) in the rat pulmonary artery and may contribute to Ca2+-sensitization and contraction via rho-kinase translocation and phosphorylation of MYPT-1.
Figures
Figure 1
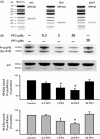
Expression of src family kinases in intra-pulmonary artery. (A) mRNA expression by polymerase chain reaction for src, fyn, and yes. The examples shown are representative of triplicate determinations. Thirty-two polymerase chain reaction cycles were carried out for each cDNA. Values in parentheses indicate expected amplicon sizes in base pairs (bp). (B) Protein expression of src family kinases by western blot analysis of intra-pulmonary artery. Three bands (two at ∼59/60 kDa, and one at 54 kDa) were visualized with anti-src and by anti-phospho-src family kinases (tyr-416). Phospho-src family kinases (tyr-416) immunoreactivity at 60 and 54 kDa was inhibited by PP2 (3 µM, *P < 0.05, n = 8 rats and 30 µM, P < 0.05, n = 8 rats) but not by PP3 (30 µM, n = 8 rats).
Figure 2
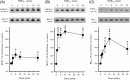
Effect of src family kinase inhibitors on PGF2α-induced contraction in α-toxin-permeabilized intra-pulmonary artery. intra-pulmonary arteries were pre-constricted with pCa6.9 for 30 min, prior to the addition of 100 µM PGF2α for 40 min (A_–_C) or 90 min (D). Compared with appropriate time controls (n = 12 arteries), this contraction was significantly relaxed by SU6656 [(A) 3 µM, 28 ± 3%, *P < 0.05, n = 8 arteries and 30 µM, 54 ± 4%, *P < 0.01, n = 8 arteries] and by PP2 [(B) 3 µM, 25 ± 5%, *P < 0.05, n = 8 arteries and 30 µM, 46 ± 5%, *P < 0.01, n = 12 arteries]. PP3 also caused relaxation at 30 µM [(C) 28 ± 2%, *P < 0.05, n = 12 arteries] but was significantly less effective than PP2 (†P < 0.05). 2-Bromopalmitate (2-BP) caused a transient contraction (non-significant) followed by significant relaxation [(D) 100 µM, 71 ± 4%, *P < 0.01, n = 7 arteries, compared with time controls, n = 7 arteries].
Figure 3
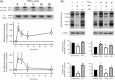
Effects of PGF2α on src family kinases tyr-416 auto-phosphorylation and tyrosine phosphorylation of multiple proteins in intra-pulmonary artery. (A) PGF2α-enhanced phospho-src (tyr-416) immunoreactivity at 60 and 54 kDa in a time-dependent manner (20 µM, *P < 0.05, n = 10–12 rats). (B) PGF2α also enhanced phospho-tyrosine immunoreactivity at multiple protein bands (20 µM, *P < 0.01, n = 10–15 rats). This was inhibited by PP2 (30 µM, †P < 0.05 vs. PGF2α, n = 10–15 rats) but not by PP3 (30 µM, n = 12 rats). Data are expressed as the ratio of phospho-tyrosine/β-actin immunoreactivity.
Figure 4
Effects of PGF2α on phosphorylation of MYPT-1 (120–130 kDa) and MLC20 (18 kDa) in intra-pulmonary artery. Phosphorylation was enhanced on MYPT-1 at thr-697 [(A) *P < 0.05, n = 6–8 rats] and thr-855 [(B) *P < 0.05; **P < 0.01, n = 6–10 rats] and on MLC20 at ser-19 [(C) *P < 0.05; **P < 0.01, n = 6–11 rats].
Figure 5
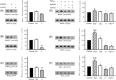
Effects of inhibition of src family kinases and rho-kinase on MYPT-1 (120–130 kDa) and MLC20 (18 kDa) phosphorylation. [(A)–(C)] In the absence of PGF2α, phosphorylation of all three sites was not affected by SU6656 (30 µM, n = 7 rats), but variably inhibited by Y27632 (10 µM, *P < 0.05, n = 8 rats). [(D)–(F)] In the presence of PGF2α, phosphorylation of all three sites was enhanced (*P < 0.05; **P < 0.01, n = 8–11 rats), and this enhancement was inhibited by SU6656 (30 µM, MYPT-1 at thr-855 and MLC20 at ser-19, †P < 0.01, n = 8 rats), Y27632 (10 µM, †P < 0.01, n = 9 rats), and the two drugs in combination (†P < 0.01, n = 8 rats). No additive effect of SU6656 and Y27632 was apparent.
Figure 6
Interaction between src family kinases and rho-kinase activity in PGF2α-stimulated intra-pulmonary artery and pulmonary artery smooth muscle cells. (A) In intra-pulmonary artery, in the absence of PGF2α, the pCa6.9 contraction was inhibited by Y27632 (10 µM, *P < 0.001, n = 8, compared with time controls, n = 8 arteries) but not by SU6656 (30 µM, n = 8 arteries). (B) Y27632 also inhibited contraction in the presence of PGF2α (10 µM, 84 ± 2%, *P < 0.001, n = 8 arteries) and this relaxation was unaltered by the additional presence of SU6656 (30 µM, 87 ± 2%, *P < 0.001, n = 8 arteries). The effect of SU6656 alone (from Figure 2) is included as a comparison. (C) In pulmonary artery smooth muscle cells, PGF2α triggered translocation of rho-kinase (ROCK-2) immunoreactivity from the nucleus to the cytoskeleton (20 µM, *P < 0.05, n = 6 determinations) and this was prevented by SU6656 (30 µM; *P < 0.05, n = 6 determinations).
Similar articles
- Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery.
Knock GA, Snetkov VA, Shaifta Y, Drndarski S, Ward JP, Aaronson PI. Knock GA, et al. Cardiovasc Res. 2008 Dec 1;80(3):453-62. doi: 10.1093/cvr/cvn209. Epub 2008 Aug 5. Cardiovasc Res. 2008. PMID: 18682436 Free PMC article. - ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: Role of Src-family kinases and ARHGEF1.
MacKay CE, Shaifta Y, Snetkov VV, Francois AA, Ward JPT, Knock GA. MacKay CE, et al. Free Radic Biol Med. 2017 Sep;110:316-331. doi: 10.1016/j.freeradbiomed.2017.06.022. Epub 2017 Jul 1. Free Radic Biol Med. 2017. PMID: 28673614 Free PMC article. - Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca(2+) sensitization.
Knock GA, Snetkov VA, Shaifta Y, Connolly M, Drndarski S, Noah A, Pourmahram GE, Becker S, Aaronson PI, Ward JP. Knock GA, et al. Free Radic Biol Med. 2009 Mar 1;46(5):633-42. doi: 10.1016/j.freeradbiomed.2008.11.015. Epub 2008 Dec 6. Free Radic Biol Med. 2009. PMID: 19103285 Free PMC article. - Rho-Mancing to Sensitize Calcium Signaling for Contraction in the Vasculature: Role of Rho Kinase.
Szasz T, Webb RC. Szasz T, et al. Adv Pharmacol. 2017;78:303-322. doi: 10.1016/bs.apha.2016.09.001. Epub 2016 Oct 27. Adv Pharmacol. 2017. PMID: 28212799 Review. - Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease.
MacKay CE, Knock GA. MacKay CE, et al. J Physiol. 2015 Sep 1;593(17):3815-28. doi: 10.1113/jphysiol.2014.285304. Epub 2014 Dec 1. J Physiol. 2015. PMID: 25384773 Free PMC article. Review.
Cited by
- Tyrosine kinases as key modulators of smooth muscle function in health and disease.
Knock GA. Knock GA. J Physiol. 2015 Sep 1;593(17):3805-6. doi: 10.1113/JP271023. J Physiol. 2015. PMID: 26331833 Free PMC article. No abstract available. - Sildenafil improves hemodynamic changes caused by acute pulmonary embolism by inhibiting Rho kinase activity.
Wang H, Li W, Yan Y, Shi MJ, Hou F, Zhang RP. Wang H, et al. J Int Med Res. 2024 Apr;52(4):3000605241240938. doi: 10.1177/03000605241240938. J Int Med Res. 2024. PMID: 38603613 Free PMC article. - Hypoxic pulmonary vasoconstriction in isolated rat pulmonary arteries is not inhibited by antagonists of H2 S-synthesizing pathways.
Prieto-Lloret J, Shaifta Y, Ward JP, Aaronson PI. Prieto-Lloret J, et al. J Physiol. 2015 Jan 15;593(2):385-401. doi: 10.1113/jphysiol.2014.277046. J Physiol. 2015. PMID: 25630260 Free PMC article. - Enhancement of myofilament calcium sensitivity by acute hypoxia in rat distal pulmonary arteries.
Weigand L, Shimoda LA, Sylvester JT. Weigand L, et al. Am J Physiol Lung Cell Mol Physiol. 2011 Sep;301(3):L380-7. doi: 10.1152/ajplung.00068.2011. Epub 2011 Jun 10. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21665962 Free PMC article. - Thyroxine Induces Acute Relaxation of Rat Skeletal Muscle Arteries via Integrin αvβ3, ERK1/2 and Integrin-Linked Kinase.
Selivanova EK, Gaynullina DK, Tarasova OS. Selivanova EK, et al. Front Physiol. 2021 Sep 14;12:726354. doi: 10.3389/fphys.2021.726354. eCollection 2021. Front Physiol. 2021. PMID: 34594239 Free PMC article.
References
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. - PubMed
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. - PubMed
- Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem. 1996;271:4733–4740. - PubMed
- Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr-850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous