The G2 p38-mediated stress-activated checkpoint pathway becomes attenuated in transformed cells - PubMed (original) (raw)
The G2 p38-mediated stress-activated checkpoint pathway becomes attenuated in transformed cells
Alexei Mikhailov et al. Curr Biol. 2007.
Abstract
When human cells are stressed during G2, they are delayed from entering mitosis via a checkpoint mediated by the p38 kinase, and this delay can be modeled by the selective activation of p38 with anisomycin. Here, we report, on the basis of live-cell studies, that 75 nM anisomycin transiently (1 hr) activates p38 which, in turn, rapidly and completely blocks entry into mitosis for at least 4 hr in all primary, telomerase- or spontaneously immortalized (p53+ and pRB+) human cells. However, the same treatment does not delay entry into mitosis in cancer cells, or the delay in entering mitosis is shortened, even though it induces a similar transient and comparable (or stronger) activation of p38. Because the primary substrate of p38, the MK2 kinase, is also transiently (1-2 hr) activated by anisomycin in both normal and cancer cells, checkpoint disruption in transformed cells occurs downstream of MK2. Finally, observations on isogenic lines reveal that the duration of the stress checkpoint is shortened in cells lacking both p53 and pRb and that the constitutive expression of an active H-Ras oncogene in these cells further attenuates the checkpoint via an ERK1/2-dependent manner. Thus, transformation leads to attenuation of the p38-mediated stress checkpoint. This outcome is likely selected for during transformation because it confers the ability to outgrow normal cells under stressful in vitro (culture) or in vivo (tumor) environments. Our data caution against using cancer cells to study how p38 produces a G2 arrest.
Figures
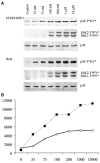
Figure 1. Seventy-Five Nanomolars of Anisomycin Strongly Activates p38, but Not JNK, in Both Normal and Cancer Cells in a Dose-Dependent Fashion
(A) A 30 min treatment with nanomolar concentrations of anisomycin activates p38 in telomerase-immortalized (hTERT-RPE1) cells, as well as in fully oncogenic (HeLa) cells. Both p38 and JNK1 and 2 activation were examined in western blots with antibodies against dually phosphorylated and activated forms of p38 (p38-T*GY*) and JNK1 and 2 (JNK1 and 2-T*PY*). Equal amounts of protein per gel lane were loaded. Total p38 antibody blotting (p38) is the loading control. (B) The band densities in (A) were measured so that a plot of p38 phosphorylation (y axis, arbitrary units) versus anisomycin concentration (x axis, nM) could be produced. Note that the amount of p38 phosphorylated in HeLa (dotted line) is significantly higher at all anisomycin concentrations than in RPE1 (solid line).
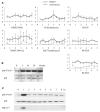
Figure 2. Activating p38 with Anisomycin Inhibits Entry into Mitosis in Normal but Not Cancer Cells
(A and B) Graphic representations from live-cell population studies, depicting the percentage of total cells within the same microscopic field that entered mitosis (y axis) every half hour (x axis) in the absence of p38 activation (solid line, open squares; DMSO only) and after (gray line, solid squares) the activation of p38 at 0 time with 75 nM anisomycin. The percentages represent the average from several experiments (for n, see Table S1) ± the standard error of the mean (SEM). Typically, 7 min elapsed between the addition of the drug to the initiation of recordings. Note that the activation of p38 rapidly and completely inhibits entry into mitosis (the G2-M transition) for at least 4 hr in normal cells (A) but to a much lesser extent in tumor cells (B). (C) The behavior of normal (RPE1) and cancer (HeLa) cells after the activation of p38 with anisomycin during late G2. RPE1 (top) and HeLa (bottom) cells in the early stages of chromosome condensation (arrows at 0 and 10 min, top panel) were located within a culture and followed by timelapse imaging. Ten minutes into imaging, the cultures were perfused with 75 nM anisomycin (see the Supplemental Experimental Procedures). The activation of p38 in RPE1 cells rapidly arrests progression through G2 and reverses chromosome condensation (compare chromosomes at 20 versus 10 min, top panel, arrows). In contrast, after the activation of p38 in HeLa cells (bottom panel), chromosome condensation continued normally, and nuclear-envelope breakdown (NEB) occurred, as in nontreated controls, ∼50 min later. The scale bar represents 10 μM.
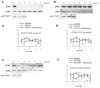
Figure 3. Attenuation of the p38-Mediated Delay in Entering Mitosis Occurs during Oncogenic Transformation
(A) Graphic representations from population studies (described in Figure 1) depicting the rate at which cells enter mitosis in cultures of immortalized mammary epithelia that contain (HMEC184A1) or lack (HMEC184AA2) a functional p53. Similar analyses were conducted on human keratinocytes lacking functional p53 (E6) or lacking both p53 and pRB (E6E7), as well as in hTERT-immortalized BJ fibroblasts containing (BJ-EN) or lacking (BJ-ELB) p53 and pRB or lacking p53 and pRB but expressing V12Ras (BJ-ELR). In general, the sustainability (duration) of the G2 delay induced by the activation of p38 decreases with increasing degrees of transformation. The percentages represent the average from several experiments (for n, see Table S1) ± the SEM. (B) Western blot depicting dually phosphorylated and activated p38 (p38-T*GY*) versus total p38 loading control (bottom panel) in BJ-ELR cells. Samples were prepared 30 min after the incubation of cultures in media plus DMSO FOR 30 min or for various times in media containing 75 nM anisomycin (dissolved in DMSO). (C) Similar to (B) except these blots show the activity of p38 as well as its downstream target, MK2, over 8 hr. Note that in response to anisomycin, both p38 and MK2 are strongly activated in BJ-ELR cells within the first hour but both then decline to near pretreatment levels during the second hour.
Figure 4. ERK1 and 2 Activity Participates in Attenuating the p38-Mediated G2 Delay in BJ-ELR Cells
(A) Western blot showing that a 3 hr treatment with 400 μM U0126 completely inhibits ERK1 and 2 activity in BJ-ELR cells and, although it also initially depresses p38 activity, p38 activity returns to normal levels by 4 hr. (A′) Graphic representation from population studies depicting the rate that BJ-ELR cells enter mitosis 3 hr after inhibiting ERK1 and 2 with 200 μM U0126 with (gray line, solid boxes) and without (dashed line, open triangles) a subsequent treatment (at time 0) with 75 nM anisomycin. Note that the inhibition of ERK1 and 2 before the activation of p38 leads to a complete cessation of mitotic entry in BJ-ELR cells for at least 2 hr (cf. Figure 3A). (B and B′) Same conditions as in (A) and (A′) except that U0126 was replaced by its inactive analog, U0124 (400 μM). Note that this analog neither inhibits ERK1 and 2 nor prolongs the G2 block after the activation of p38 with anisomycin (cf. Figure 4A). (C and C′) Same conditions as in (A) and (A′) except that U0126 was replaced by 600 nM CI-1040, a more potent and specific ERK1 and 2 inhibitor. The concentration of CI-1040 used in this study reduced ERK1 and 2 activity to that which approximates the level seen in normal G2 cells [25]. Under this condition, the activation of p38 with anisomycin completely inhibits entry into mitosis for at least 2 hr (cf. Figure 4A). The percentages represent the average from several experiments (for n, see Table S1) ± the SEM.
Similar articles
- The p38-mediated stress-activated checkpoint. A rapid response system for delaying progression through antephase and entry into mitosis.
Mikhailov A, Shinohara M, Rieder CL. Mikhailov A, et al. Cell Cycle. 2005 Jan;4(1):57-62. doi: 10.4161/cc.4.1.1357. Epub 2005 Jan 11. Cell Cycle. 2005. PMID: 15611649 Review. - Extracellular signal-regulated kinase 1/2 activity is not required in mammalian cells during late G2 for timely entry into or exit from mitosis.
Shinohara M, Mikhailov AV, Aguirre-Ghiso JA, Rieder CL. Shinohara M, et al. Mol Biol Cell. 2006 Dec;17(12):5227-40. doi: 10.1091/mbc.e06-04-0284. Epub 2006 Oct 11. Mol Biol Cell. 2006. PMID: 17035635 Free PMC article. - Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase.
Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ Jr. Bulavin DV, et al. Nature. 2001 May 3;411(6833):102-7. doi: 10.1038/35075107. Nature. 2001. PMID: 11333986 - Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway.
Mikhailov A, Shinohara M, Rieder CL. Mikhailov A, et al. J Cell Biol. 2004 Aug 16;166(4):517-26. doi: 10.1083/jcb.200405167. Epub 2004 Aug 9. J Cell Biol. 2004. PMID: 15302851 Free PMC article. - p38 and Chk1 kinases: different conductors for the G(2)/M checkpoint symphony.
Bulavin DV, Amundson SA, Fornace AJ. Bulavin DV, et al. Curr Opin Genet Dev. 2002 Feb;12(1):92-7. doi: 10.1016/s0959-437x(01)00270-2. Curr Opin Genet Dev. 2002. PMID: 11790561 Review.
Cited by
- Mitosis in vertebrates: the G2/M and M/A transitions and their associated checkpoints.
Rieder CL. Rieder CL. Chromosome Res. 2011 Apr;19(3):291-306. doi: 10.1007/s10577-010-9178-z. Chromosome Res. 2011. PMID: 21194009 Review. - Tubulin mRNA stability is sensitive to change in microtubule dynamics caused by multiple physiological and toxic cues.
Gasic I, Boswell SA, Mitchison TJ. Gasic I, et al. PLoS Biol. 2019 Apr 9;17(4):e3000225. doi: 10.1371/journal.pbio.3000225. eCollection 2019 Apr. PLoS Biol. 2019. PMID: 30964857 Free PMC article. - Low-dose anisomycin sensitizes melanoma cells to TRAIL induced apoptosis.
Slipicevic A, Øy GF, Rosnes AK, Stakkestad Ø, Emilsen E, Engesæter B, Mælandsmo GM, Flørenes VA. Slipicevic A, et al. Cancer Biol Ther. 2013 Feb;14(2):146-54. doi: 10.4161/cbt.22953. Epub 2012 Nov 28. Cancer Biol Ther. 2013. PMID: 23192275 Free PMC article. - A prognostic signature of G(2) checkpoint function in melanoma cell lines.
Omolo B, Carson C, Chu H, Zhou Y, Simpson DA, Hesse JE, Paules RS, Nyhan KC, Ibrahim JG, Kaufmann WK. Omolo B, et al. Cell Cycle. 2013 Apr 1;12(7):1071-82. doi: 10.4161/cc.24067. Epub 2013 Mar 1. Cell Cycle. 2013. PMID: 23454897 Free PMC article. - Circumventing photodamage in live-cell microscopy.
Magidson V, Khodjakov A. Magidson V, et al. Methods Cell Biol. 2013;114:545-60. doi: 10.1016/B978-0-12-407761-4.00023-3. Methods Cell Biol. 2013. PMID: 23931522 Free PMC article. Review.
References
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. - PubMed
- Michea L, Ferguson DR, Peters EM, Andrews PM, Kirby MR, Burg MB. Cell cylce delay and apoptosis are induced by high salt and urea in renal medullary cells. Am. J. Physiol. Renal Physiol. 2000;278:F209–F218. - PubMed
- Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ. Initiation of a G2/M checkpoint after ultraviolet radiation requries p38 kinase. Nature. 2001;411:102–107. - PubMed
- Zhang Y, Song S, Fong C-C, Tsang C-H, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J. Invest. Dermatol. 2003;120:849–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM040198-21/GM/NIGMS NIH HHS/United States
- 40198/PHS HHS/United States
- R01 DE015935/DE/NIDCR NIH HHS/United States
- R37 GM040198/GM/NIGMS NIH HHS/United States
- DE015935/DE/NIDCR NIH HHS/United States
- R37 GM040198-24/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous