SUMOylation of pontin chromatin-remodeling complex reveals a signal integration code in prostate cancer cells - PubMed (original) (raw)
SUMOylation of pontin chromatin-remodeling complex reveals a signal integration code in prostate cancer cells
Jung Hwa Kim et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Posttranslational modification by small ubiquitin-like modifier (SUMO) controls diverse cellular functions of transcription factors and coregulators and participates in various cellular processes including signal transduction and transcriptional regulation. Here, we report that pontin, a component of chromatin-remodeling complexes, is SUMO-modified, and that SUMOylation of pontin is an active control mechanism for the transcriptional regulation of pontin on androgen-receptor target genes in prostate cancer cells. Biochemical purification of pontin-containing complexes revealed the presence of the Ubc9 SUMO-conjugating enzyme that underlies its function as an activator. Intriguingly, 5alpha-dihydroxytestosterone treatments significantly increased the SUMOylation of pontin, and SUMOylated pontin showed further activation of a subset of nuclear receptor-dependent transcription and led to an increase in proliferation and growth of prostate cancer cells. These data clearly define a functional model and provide a link between SUMO modification and prostate cancer progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
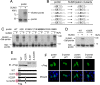
Purification of pontin-containing complex. (A) The pontin-containing complex was immunoprecipitated with anti-Flag IgG-conjugated agarose beads from 293T cell extracts, and the bound proteins were eluted with Flag peptide and resolved by SDS/PAGE. (B) Peptide sequences of pontin-associated polypeptides from LC-MS/MS analysis. (C) Western blot analysis was performed by using the indicated antibodies, and reptin, β-catenin, p400, and Ubc9 were detected in the eluates. (D) Coimmunoprecipitation of endogenous pontin with Ubc9. Cell lysates were subjected to immunoprecipitation with either anti-Ubc9 IgG or control IgG, and the resultant precipitates were subjected to immunoblotting with anti-pontin IgG.
Fig. 2.
Lysine 225 of pontin is crucial for SUMO modification. (A) In vitro modification of pontin by SUMO. 35S-labeled _in vitro_-translated pontin was incubated in a SUMOylation mix containing purified E1, E2, and ATP in the absence or presence of SUMO. (B) Search for consensus site (ψ KxE) for SUMOylation in pontin, where ψ is an aliphatic amino acid, and K is the lysine conjugated to SUMO. (C) Lysine 225 of pontin is a major SUMO conjugation site. In vitro SUMOylation assay was conducted with 35S-labeled _in vitro_-translated Gal4-fused wild type or the K225R mutant of pontin as in A. (D) 293T cells were cotransfected with plasmids expressing either Gal4-fused wild type or the K225R mutant of pontin in the presence of SUMO and Ubc9. Western blotting was performed with anti-Gal4 antibody. (E) Immunoblot analysis indicates expression of Flag-tagged pontin, SUMO-fused pontin, pontin K225R, or SUMO-fused pontin K225R and their schematic representations. (F) Subcellular localization of Flag-tagged pontin, SUMO-fused pontin, pontin K225R, or SUMO-fused pontin K225R in HeLa cells (green). Nuclei were visualized by DAPI staining (blue).
Fig. 3.
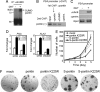
SUMOylation of pontin is required for transcriptional activation of androgen-receptor target genes. (A) Knockdown of pontin diminishes the PSA, KLK2, and NKX3.1 transcripts in the presence of DHT in LNCaP cells. (B) Fold change of AR target gene KLK2 transcripts after introduction of pontin K225R or SUMO-fused pontin K225R. (C–E) SUMOylation of pontin enhances the transcriptional activation function of pontin. Luciferase assay was conducted after cotransfection of ARE-luciferase reporter in the presence of DHT (C). Expression of pontin activated an RORα 2E-luciferase reporter (D) but not a RARE-luciferase reporter (E). (F) In vivo association experiments between pontin and nuclear receptors in 293T cells. (G) Coimmunoprecipitation assay to verify interaction of Flag-tagged pontin or pontin K225R with β-catenin, CBP, or Tip60. Coimmunoprecipitation assay was performed with anti-Flag IgG, and precipitated materials were detected with either β-catenin, CBP, or Tip60 antibody, respectively.
Fig. 4.
SUMOylation of pontin increases the proliferation and growth of prostate cancer cells. (A) SUMOylated pontin is increased in LNCaP cells in the presence of DHT. (B) Two-step ChIP assay with anti-pontin and anti-SUMO IgGs indicates that SUMO-modified pontin is present on the PSA promoter under activation condition. (C) ChIP analysis of Ubc9 on the PSA promoter with DHT treatment for 1 h in LNCaP cells. (D) AR target genes PSA and KLK2 transcripts after introduction of either shRNA against Ubc9 or nonspecific shRNA. (E) Proliferation curves of mock-, pontin-K225R-, SUMO-pontin-, or SUMO-pontin K225R-expressing LNCaP cells. Values are represented as mean ± SD of three independent experiments. (F) The anchorage-independent growth of LNCaP cells expressing pontin-, pontin-K225R-, SUMO-pontin-, or SUMO-pontin K225R in soft agar. Representative image is shown for each group.
Similar articles
- Site-specific inhibition of the small ubiquitin-like modifier (SUMO)-conjugating enzyme Ubc9 selectively impairs SUMO chain formation.
Wiechmann S, Gärtner A, Kniss A, Stengl A, Behrends C, Rogov VV, Rodriguez MS, Dötsch V, Müller S, Ernst A. Wiechmann S, et al. J Biol Chem. 2017 Sep 15;292(37):15340-15351. doi: 10.1074/jbc.M117.794255. Epub 2017 Aug 7. J Biol Chem. 2017. PMID: 28784659 Free PMC article. - Dynamin interacts with members of the sumoylation machinery.
Mishra RK, Jatiani SS, Kumar A, Simhadri VR, Hosur RV, Mittal R. Mishra RK, et al. J Biol Chem. 2004 Jul 23;279(30):31445-54. doi: 10.1074/jbc.M402911200. Epub 2004 Apr 30. J Biol Chem. 2004. PMID: 15123615 - Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity.
Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L. Sentis S, et al. Mol Endocrinol. 2005 Nov;19(11):2671-84. doi: 10.1210/me.2005-0042. Epub 2005 Jun 16. Mol Endocrinol. 2005. PMID: 15961505 - SUMO: getting it on.
Anckar J, Sistonen L. Anckar J, et al. Biochem Soc Trans. 2007 Dec;35(Pt 6):1409-13. doi: 10.1042/BST0351409. Biochem Soc Trans. 2007. PMID: 18031233 Review. - Sumoylation and Its Contribution to Cancer.
Lee JS, Choi HJ, Baek SH. Lee JS, et al. Adv Exp Med Biol. 2017;963:283-298. doi: 10.1007/978-3-319-50044-7_17. Adv Exp Med Biol. 2017. PMID: 28197919 Review.
Cited by
- Cytoplasmic expression of pontin in renal cell carcinoma correlates with tumor invasion, metastasis and patients' survival.
Zhang X, Ren J, Yan L, Tang Y, Zhang W, Li D, Zang Y, Kong F, Xu Z. Zhang X, et al. PLoS One. 2015 Mar 9;10(3):e0118659. doi: 10.1371/journal.pone.0118659. eCollection 2015. PLoS One. 2015. PMID: 25751257 Free PMC article. - The emergence of the conserved AAA+ ATPases Pontin and Reptin on the signaling landscape.
Rosenbaum J, Baek SH, Dutta A, Houry WA, Huber O, Hupp TR, Matias PM. Rosenbaum J, et al. Sci Signal. 2013 Mar 12;6(266):mr1. doi: 10.1126/scisignal.2003906. Sci Signal. 2013. PMID: 23482663 Free PMC article. - Multi-modulation of nuclear receptor coactivators through posttranslational modifications.
Han SJ, Lonard DM, O'Malley BW. Han SJ, et al. Trends Endocrinol Metab. 2009 Jan;20(1):8-15. doi: 10.1016/j.tem.2008.10.001. Epub 2008 Nov 18. Trends Endocrinol Metab. 2009. PMID: 19019695 Free PMC article. Review. - Diagnostic and prognostic values of tissue hsa-miR-30c and hsa-miR-203 in prostate carcinoma.
Huang Z, Zhang L, Yi X, Yu X. Huang Z, et al. Tumour Biol. 2016 Apr;37(4):4359-65. doi: 10.1007/s13277-015-4262-9. Epub 2015 Oct 24. Tumour Biol. 2016. PMID: 26499781 - The impact of dysregulation SUMOylation on prostate cancer.
Li K, Wang H, Jiang B, Jin X. Li K, et al. J Transl Med. 2025 Mar 6;23(1):286. doi: 10.1186/s12967-025-06271-2. J Transl Med. 2025. PMID: 40050932 Free PMC article. Review.
References
- Baek SH, Rosenfeld MG. Biochem Biophys Res Commun. 2004;319:707–714. - PubMed
- Feldman BJ, Feldman D. Nat Rev Cancer. 2001;1:34–45. - PubMed
- Berger SL. Nature. 2007;447:407–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous