Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis - PubMed (original) (raw)
Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis
Kirsteen H Maclean et al. J Clin Invest. 2008 Jan.
Erratum in
- J Clin Invest. 2008 Apr;118(4):1584
Abstract
Despite great interest in cancer chemoprevention, effective agents are few. Here we show that chloroquine, a drug that activates the stress-responsive Atm-p53 tumor-suppressor pathway, preferentially enhances the death of Myc oncogene-overexpressing primary mouse B cells and mouse embryonic fibroblasts (MEFs) and impairs Myc-induced lymphomagenesis in a transgenic mouse model of human Burkitt lymphoma. Chloroquine-induced cell death in primary MEFs and human colorectal cancer cells was dependent upon p53, but not upon the p53 modulators Atm or Arf. Accordingly, chloroquine impaired spontaneous lymphoma development in Atm-deficient mice, a mouse model of ataxia telangiectasia, but not in p53-deficient mice. Chloroquine treatment enhanced markers of both macroautophagy and apoptosis in MEFs but ultimately impaired lysosomal protein degradation. Interestingly, chloroquine-induced cell death was not dependent on caspase-mediated apoptosis, as neither overexpression of the antiapoptotic protein Bcl-2 nor deletion of the proapoptotic Bax and Bak affected chloroquine-induced MEF death. However, when both apoptotic and autophagic pathways were blocked simultaneously, chloroquine-induced killing of Myc-overexpressing cells was blunted. Thus chloroquine induces lysosomal stress and provokes a p53-dependent cell death that does not require caspase-mediated apoptosis. These findings specifically demonstrate that intermittent chloroquine use effectively prevents cancer in mouse models of 2 genetically distinct human cancer syndromes, Burkitt lymphoma and ataxia telangiectasia, suggesting that agents targeting lysosome-mediated degradation may be effective in cancer prevention.
Figures
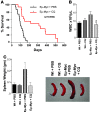
Figure 1. CQ prevents Myc-induced lymphomagenesis.
(A) Administration (i.p.) of CQ impairs lymphoma development in Eμ-Myc transgenic mice. Beginning at weaning, Eμ-Myc mice were treated with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone (n = 18 for each group). Median survival time was 98 days for mice treated with PBS versus 265 days for mice receiving CQ (P = 0.0002). (B) CQ treatment prevents lymphocytosis in precancerous Eμ-Myc transgenic mice. Beginning at 4 weeks, Eμ-Myc transgenic mice and their wild-type littermates (n = 5–8 for each group) were injected with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone. At 7 weeks, mice were then analyzed for wbc counts. CQ had no effect on wbc numbers in wild-type mice but had profound effects on wbc numbers in Eμ-Myc mice. *P < 0.05. (C) CQ treatment prevents splenomegaly in precancerous Eμ-Myc transgenic mice. Beginning at 4 weeks, Eμ-Myc transgenic mice and their wild-type littermates (n = 8 for each group) were injected with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or injected with PBS alone. At 7 weeks, mice were then analyzed for spleen weight. Pictures in inset show representative spleens from wild-type and Eμ-Myc transgenic mice injected with PBS or CQ.
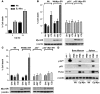
Figure 2. Myc augments p53-dependent, CQ-induced cell death.
(A) CQ augments cell death in Eμ-Myc transgenic B cells. B cells cultured from 4-week-old nontransgenic and Eμ-Myc transgenic mice were treated with 50 μM CQ for 24 h. The percentage of viable cells was determined by propidium iodide incorporation. Results shown are the mean of 3 independent B cell cultures. (B) p53 is required for sensitization of Myc-expressing MEFs to CQ. _Myc_-ERTAM–expressing MEFs were either left untreated (Unt) or were treated for 24 h with 4-HT alone or with 4-HT and 50 μM CQ. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. Western blot analyses of the indicated cells demonstrated equal levels of expression of the _Myc_-ERTAM transgene. (C) Myc sensitizes HCT116 colon cancer cells to p53-dependent, CQ-induced death. Cells were treated for 24 h with 4-HT and were either left untreated or were treated with 50 μM CQ for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. (D) CQ treatment leads to clearance of Arf/p53/Puma-expressing precancerous Eμ-Myc B cells. Beginning at 4 weeks, Eμ-Myc transgenic mice and their wild-type littermates were injected with 3.5 mg/kg CQ (in PBS) i.p. every 5 days or with PBS alone. At 7 weeks, mice were sacrificed and B220+ B cells analyzed. As controls, lysates were also prepared from primary _Arf_–/– and _p53_–/– B cells. Protein extracts were prepared and evaluated for their levels of the indicated antibodies.
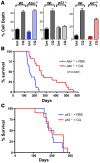
Figure 3. CQ is a p53-dependent chemoprevention agent.
(A) CQ induces cell death in wild-type, Atm- and _Arf-_deficient MEFs, but not in _p53-_deficient MEFs. Early passage (p2) MEFs paired wild-type, _Atm_–/–, _Arf_–/–, and _p53_–/– MEFs were left untreated or were treated with CQ (50 μM) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. (B) Administration of CQ i.p. impairs thymoma development in _Atm_-deficient mice. Beginning at weaning, _Atm_–/– mice were treated with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone (n = 20 for each group). Median survival time was 147 days for mice treated with PBS versus 254 days for mice receiving CQ (P < 0.0001). (C) Administration of CQ i.p. does not affect thymoma development in _p53_-deficient mice. At weaning, _p53_–/– mice were treated with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone (n = 20 for each group). Median survival time was 198 days for mice in both groups.
Figure 4. CQ induces hallmarks of apoptosis, yet can induce cell death independent of apoptosis.
(A) CQ induces hallmarks of apoptosis in Myc-expressing MEFs. Early passage MEFs were treated for 24 h with 4-HT to activate _Myc_-ERTAM and then treated with or without CQ (50 μM) for 24 h. Western blot analyses of the indicated cells demonstrated cleavage of caspase-9 and the caspase substrate PARP; asterisks indicate the specific cleavage products. (B) CQ-induced cell death is not inhibited by the broad-spectrum caspase inhibitor zVAD-fmk. Early passage MEFs were pretreated with zVAD-fmk for 1 h prior to being treated with CQ (50 μM) for 24 h. Myc-overexpressing MEFs were also pretreated with zVAD-fmk for 1 h and either serum starved (0.1%) or treated with CQ (50 μM) for a further 24 h. Viability was determined by propidium iodide staining. Results shown are the mean of 2 independent experiments. (C) CQ-induced cell death is not inhibited by Bcl-2 or Bcl-XL. Primary bone marrow B cell cultures derived from Eμ-Myc transgenic mice were infected with MSCV-IRES-GFP, MSCV–_Bcl-2_–IRES-GFP or MSCV–_Bcl-XL_–IRES–GFP retroviruses. GFP+ cells were isolated and expanded in culture and then were treated with CQ (50 μM) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. By 24 h, CQ induced a 2.1-, 3.1-, or 7-fold increase in the death of GFP-, Bcl-2–, or Bcl-XL–expressing Eμ-Myc B cells, respectively. (D) CQ induces cell death in _Bax/Bak_–/– MEFs. Early passage MEFs were treated with or without CQ (50 μM) for 24 h. The percentage cell death was determined by propidium iodide incorporation. Results shown are the mean of 3 independent experiments.
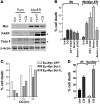
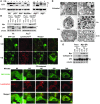
Figure 5. CQ provokes markers of macroautophagy yet inhibits lysosomal functions.
(A) CQ induces the accumulation of PE-modified LC3. Early passage (p2) MEFs were treated with 50 μM CQ for 24 h. Expression and modification of LC3 (pro-form, LC3-I, and PE-modified LC3-II) was analyzed by western blot. (B) Indicated MEFs were treated for 24 h with 4-HT to activate _Myc_-ERTAM and with or without 50 μM CQ for 24 h. Expression and modification of LC3 were monitored by western blot analyses. (C) Top: GFP-LC3B–expressing MEFs were incubated with 100 nM Lysotracker for 30 min with or without CQ (50 μM) for 6 h. Cells were then imaged using a Nikon inverted confocal fluorescent microscope. Bottom: Time course analyses of CQ-induced changes in GFP-LC3B–expressing MEFs. Cells were incubated with 100 nM Lysotracker for 30 min, then treated with CQ (50 μM), followed by real-time video microscopy (see Supplemental Videos 1 and 2). Images were taken at the indicated times using a Nikon inverted confocal fluorescent microscope. Original magnification, ×63. (D) Wild-type early passage (p2) MEFs were treated with or without CQ (50 μM) for 4 h or 24 h. Cells were fixed with 2% (vol/vol) glutaraldehyde, and 1-μM sections were analyzed by transmission electron microscopy. Magnification, ×5,000. Scale bars: 1 μM. L, lysosome; A, autophagosome; AL, autophagolysosome. (E) CQ induces the accumulation of p62 and cathepsin D. MEFs were treated for 24 h with 4-HT to activate _Myc_-ERTAM and then treated with or without CQ for 24 h. Expression of p62 and cathepsin D was analyzed by western blot. *NS, nonspecific.
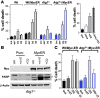
Figure 6. CQ-induced death is impaired by blockade of both apoptosis and autophagy.
(A) CQ can induce cell death in the absence of autophagy. Left: Early passage (p2) wild-type and _Atg7_-deficient MEFs expressing MSCV-IRES-puro or MSCV–IRES–_Myc_-ERTAM–puro retroviruses were treated for 24 h with 4-HT to activate _Myc_-ERTAM and were then left untreated or were treated with 50 μM CQ for 24 h or serum starved (SS; 0.1% FBS) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. Right: Wild-type MEFs were treated with either 3-MA alone (2 mM) or in combination with CQ (50 μM) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. (B) Blockade of autophagy does not prevent CQ from inducing hallmarks of apoptosis. _Atg7_-deficient MEFs expressing MSCV-IRES-puro or MSCV–IRES–_Myc_-ERTAM–puro retroviruses were treated for 24 h with 4-HT to activate _Myc_-ERTAM and were then left untreated or were treated with 50 μM CQ for 24 h. Cleavage of the caspase-3 substrate PARP was detected by western blot analysis in Myc-expressing _Atg7_-deficient MEFs. The asterisk indicates the specific cleavage product. (C) CQ-induced cell death is prevented by the combined blockade in autophagy and apoptosis. Early passage (p2) wild-type and _Atg7_-deficient MEFs expressing the MSCV–IRES–_Myc_-ERTAM–puro retrovirus were pretreated with qVD-fmk (CI; 20 μM) for 1 h prior to being treated with CQ (50 μM) for 24 h. Viability was determined by propidium iodide staining. Results shown are the mean of 3 independent experiments.
Comment in
- Antimalarial therapy prevents Myc-induced lymphoma.
Dang CV. Dang CV. J Clin Invest. 2008 Jan;118(1):15-7. doi: 10.1172/JCI34503. J Clin Invest. 2008. PMID: 18097478 Free PMC article.
Similar articles
- Antimalarial therapy prevents Myc-induced lymphoma.
Dang CV. Dang CV. J Clin Invest. 2008 Jan;118(1):15-7. doi: 10.1172/JCI34503. J Clin Invest. 2008. PMID: 18097478 Free PMC article. - Regulation of ATM/p53-dependent suppression of myc-induced lymphomas by Wip1 phosphatase.
Shreeram S, Hee WK, Demidov ON, Kek C, Yamaguchi H, Fornace AJ Jr, Anderson CW, Appella E, Bulavin DV. Shreeram S, et al. J Exp Med. 2006 Dec 25;203(13):2793-9. doi: 10.1084/jem.20061563. Epub 2006 Dec 11. J Exp Med. 2006. PMID: 17158963 Free PMC article. - Phosphorylation of p53 serine 18 upregulates apoptosis to suppress Myc-induced tumorigenesis.
Sluss HK, Gannon H, Coles AH, Shen Q, Eischen CM, Jones SN. Sluss HK, et al. Mol Cancer Res. 2010 Feb;8(2):216-22. doi: 10.1158/1541-7786.MCR-09-0324. Epub 2010 Feb 9. Mol Cancer Res. 2010. PMID: 20145032 Free PMC article. - ATM dependent apoptosis in the nervous system.
Lee Y, McKinnon PJ. Lee Y, et al. Apoptosis. 2000 Dec;5(6):523-9. doi: 10.1023/a:1009637512917. Apoptosis. 2000. PMID: 11303911 Review. - New insights on the role of apoptosis and autophagy in HIV pathogenesis.
Gougeon ML, Piacentini M. Gougeon ML, et al. Apoptosis. 2009 Apr;14(4):501-8. doi: 10.1007/s10495-009-0314-1. Apoptosis. 2009. PMID: 19199038 Review.
Cited by
- The role of autophagy in targeted therapy for acute myeloid leukemia.
Du W, Xu A, Huang Y, Cao J, Zhu H, Yang B, Shao X, He Q, Ying M. Du W, et al. Autophagy. 2021 Oct;17(10):2665-2679. doi: 10.1080/15548627.2020.1822628. Epub 2020 Sep 22. Autophagy. 2021. PMID: 32917124 Free PMC article. Review. - Therapeutic targets in cancer cell metabolism and autophagy.
Cheong H, Lu C, Lindsten T, Thompson CB. Cheong H, et al. Nat Biotechnol. 2012 Jul 10;30(7):671-8. doi: 10.1038/nbt.2285. Nat Biotechnol. 2012. PMID: 22781696 Free PMC article. Review. - Chloroquine exposure triggers distinct cellular responses in sensitive versus resistant Plasmodium falciparum parasites.
Reiling SJ, Krohne G, Friedrich O, Geary TG, Rohrbach P. Reiling SJ, et al. Sci Rep. 2018 Jul 24;8(1):11137. doi: 10.1038/s41598-018-29422-6. Sci Rep. 2018. PMID: 30042399 Free PMC article. - Trends Analysis of Non-Hodgkin Lymphoma at the National, Regional, and Global Level, 1990-2019: Results From the Global Burden of Disease Study 2019.
Cai W, Zeng Q, Zhang X, Ruan W. Cai W, et al. Front Med (Lausanne). 2021 Sep 23;8:738693. doi: 10.3389/fmed.2021.738693. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34631756 Free PMC article. - Therapeutic Modulation of Autophagy in Leukaemia and Lymphoma.
Djavaheri-Mergny M, Giuriato S, Tschan MP, Humbert M. Djavaheri-Mergny M, et al. Cells. 2019 Jan 30;8(2):103. doi: 10.3390/cells8020103. Cells. 2019. PMID: 30704144 Free PMC article. Review.
References
- Lowe S.W., Sherr C.J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 2003;13:77–83. - PubMed
- Giaccia A.J., Kastan M.B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. - PubMed
- Kastan M.B., Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA76379/CA/NCI NIH HHS/United States
- R37 ES005777/ES/NIEHS NIH HHS/United States
- R01 CA076379/CA/NCI NIH HHS/United States
- CA21765/CA/NCI NIH HHS/United States
- R01 ES005777/ES/NIEHS NIH HHS/United States
- R01 CA071387/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- ES05777/ES/NIEHS NIH HHS/United States
- CA71387/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous