Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis - PubMed (original) (raw)
Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis
Li Yang et al. Cancer Cell. 2008 Jan.
Abstract
Aberrant TGFbeta signaling is common in human cancers and contributes to tumor metastasis. Here, we demonstrate that Gr-1+CD11b+ myeloid cells are recruited into mammary carcinomas with type II TGF beta receptor gene (Tgfbr2) deletion and directly promote tumor metastasis. Gr-1+CD11b+ cells infiltrate into the invasive front of tumor tissues and facilitate tumor cell invasion and metastasis through a process involving metalloproteinase activity. This infiltration of Gr-1+CD11b+ cells also results in increased abundance of TGF beta 1 in tumors with Tgfbr2 deletion. The recruitment of Gr-1+CD11b+ cells into tumors with Tgfbr2 deletion involves two chemokine receptor axes, the SDF-1/CXCR4 and CXCL5/CXCR2 axes. Together, these data indicate that Gr-1+CD11b+ cells contribute to TGFbeta-mediated metastasis through enhancing tumor cell invasion and metastasis.
Figures
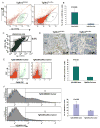
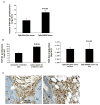
Figure 1. Gr-1+CD11b+ myeloid cells are recruited to mammary carcinomas with genetic deletion of Tgfbr2 (PyVmT/Tgfbr2MGKO)
A: Flow cytometry analysis of infiltrating Gr-1+CD11b+ cells in PyVmT/Tgfbr2MGKO tumors and PyVmT/Tgfbr2flox/flox control tumors. Shown are representative flow cytometry plots. B: Quantitative data for the presence of Gr-1+CD11b+ cells in tumors as shown in 1A. Five to seven week old mice were analyzed. C: Flow cytometry analysis of tumor-residing Gr-1+CD11b+ cells in PyVmT/Tgfbr2MGKO tumors. D: IHC of Gr-1+CD11b+ cells in the invasive front of PyVmT/Tgfbr2MGKO mammary carcinomas compared with PyVmT/Tgfbr2flox/flox tumors. Scale bars are indicated in the figures. E and F: Flow cytometry analysis of infiltrating F4/80 and VEGFR1 positive cells in PyVmT/Tgfbr2MGKO tumors and PyVmT/Tgfbr2flox/flox control tumors. Representative flow cytometry plots are shown on left and quantitative data on the right. m.g: adjacent mammary gland tissue; tu: tumor tissues. All quantitative data are presented with the mean ± standard error.
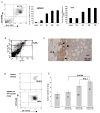
Figure 2. Gr-1+CD11b+ cells promote tumor metastasis
A: Increased Gr-1+CD11b+ cells in spleens and bone marrow of 4T1 tumor-bearing mice after tumor inoculation. Left panel: Flow cytometry analysis of Gr-1+CD11b+ cells in the spleen of tumor bearing mice 28 days following engraftment (4 or more mice per time point). Middle panel: spleen; Right panel: bone marrow (b.m.). B: Gr-1+CD11b+ cells in 4T1 tumors with flow cytometry analysis. C: IHC showing Gr-1+CD11b+ cells in the invasive front of 4T1 tumors 15 day after tumor inoculation. Scale bar, 100 μM. D: Single cell sorting of Gr-1+CD11b+ cells from tumors and spleens of 4T1 tumor-bearing mice 35 days after tumor inoculation. Flow cytometry analysis of Gr-1+CD11b+ cells after sorting is shown. E: Metastasis was significantly increased when 4T1 cells were co-injected with tumor derived Gr-1+CD11b+ cells including Gr-1+CD11b+ cells derived from tumor tissues (tu inf Gr-1+CD11b+ cells) spleens of tumor-bearing mice (tu sp Gr-1+CD11b+ cells). 4T1 cells alone and Gr-1+CD11b+ cells from normal mice (nor Gr-1+CD11b+ cells) were used as controls. Number of animals used is indicated in the bar. m.g: adjacent mammary gland tissue; tu: tumor tissues. Results are presented as the mean ± SE.
Figure 3. Gr-1+CD11b+ myeloid cells increased 4T1 tumor invasion in vivo and in vitro
A: Increased growth of recurrent tumors with 4T1 cells co-injected with Gr-1+CD11b+ cells derived from tumor tissues, or spleens of tumor-bearing mice. Number of animals is indicated in the bar. Results are presented as the mean ± SE. B: Fluorescent microscopy of 4T1 cells that invaded through a matrigel-coated transwell when co-cultured with tumor-derived Gr-1+CD11b+ cells (B-b) compared to normal Gr-1+CD11b+ cells (B-a). 4T1 cells were labeled with a green tracking dye, and Gr-1+CD11b+ cells with a red tracking dye. B-c: Close interaction between 4T1 cells and Gr-1+CD11b+ cells. Scale bar, 50 μM for all figures. C: An MMP inhibitor (GM6001, 1 mM) blocked Gr-1+CD11b+ cell promoted 4T1 cell invasion in vitro. Results are from two experiments with triplicates for each group, are presented as the mean ± SE. tu inf Gr-1+CD11b+ cells: Gr-1+CD11b+ cells from tumor tissues; tu sp Gr-1+CD11b+ cells: Gr-1+CD11b+ cells from spleens of tumor-bearing mice; nor Gr-1+CD11b+ cells: Gr-1+CD11b+ cells from spleens of normal mice. m.g: adjacent mammary gland tissue; tu: tumor tissues.
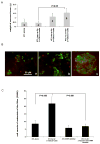
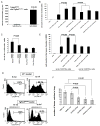
Figure 4. Increased production and function of MMPs in tumor residing Gr-1+CD11b+ cells
Real time RT-PCR of MMP14 and MMP2 (A) as well as MMP13 (B) in Gr-1+CD11b+ cells derived from tumor tissues compared with those from spleens. Results are presented as the mean ± SE. C. In situ zymography of MMPs in 4T1 mammary carcinomas and PyVmT/Tgfbr2MGKO carcinomas. Abundant green fluorescence (indicator of MMP activities) was observed in the invasive front of 4T1 tumors (C-a & c) and PyVmT/Tgfbr2MGKO carcinomas (C-d). Nuclei were labeled with 7AAD (red). Tumor sections treated with EDTA were used as negative controls (C-e). Scale bar, 50 uM for all figures. m.g: adjacent mammary gland tissue; tu: tumor tissues.
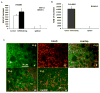
Figure 5. Increased TGFβ1 production in mammary carcinomas with genetic deletion of Tgfbr2
A: Increased TGFβ1 production in PyVmT/Tgfbr2MGKO tumors compared with PyVmT/Tgfbr2flox/flox tumors. n=4 mice per group. B left panel: Gr-1+CD11b+ cells from spleens of mice bearing large 4T1 tumors exhibited significantly higher TGFβ1 production when compared with Gr-1+CD11b+ cells from normal spleens. n=2 mice per group. B right panel: No difference of TGFβ1 production was found between tumor cell lines derived from PyVmT/Tgfbr2MGKO tumors vs PyVmT/Tgfbr2flox/flox tumors. TGFβ1 in conditioned media was measured by ELISA. Results are presented as the mean ± SE. C: Gr-1+CD11b+ cells are likely the resource for increased TGFβ13 production in mammary carcinomas with genetic deletion of Tgfbr2. IHC of TGFβ1 (left panel) showing TGFβ1 positive cells are mostly in the invasive front where Gr-1+CD11b+ cells were mostly present (right panel with Gr-1 staining). Scale bar, 50 uM for both figures. m.g: adjacent mammary gland tissue; tu: tumor tissues.
Figure 6. Mechanisms of recruitment of Gr-1+CD11b+ cells to the tumor microenvironment
A: Elevated production of CXCL5 in cell culture supernatant of PyVmT/Tgfbr2MGKO mammary carcinomas. B: In vitro migration of Gr-1+CD11b+ cells in response to CXCL5. Gr-1+CD11b+ cells migration after 6–8 hr incubation were counted and plotted. Shown is one of the representative experiments of three performed. C: Inhibition of Gr-1+CD11b+ cell recruitment to PyVmT/Tgfbr2MGKO tumors with CXCR2 antagonism. Percentage of Gr-1+CD11b+ cells in all cells from PyVmT/Tgfbr2MGKO and control tumors were plotted. Mice bearing PyVmT/Tgfbr2MGKO and control tumors were treated with a CXCR2 specific antagonist SB-265610 at 2 mg/kg/day for two weeks through I.P. D: Flow cytometry analysis of CXCR4 expression in infiltrating Gr-1+CD11b+ cells from 4T1 tumors as well as PyVmT/Tgfbr2MGKO tumors, compared with those from spleens of the same tumor-bearing mice (as labeled). Histogram of CXCR4 expression was gated on Gr-1+CD11b+ double positive cells. Shown is one of the representative mice analyzed, n=3–5 mice per group. E: In vitro migration of Gr-1+CD11b+ cells in response to SDF-1. Gr-1+CD11b+ cells migration after 6–8 hr incubation were counted and plotted. Shown is one representative experiment of three performed. F: PyVmT/Tgfbr2MGKO tumor metastasis with blockade of CXCR2 or CXCR4 alone or both. Tumor nodules in lung were counted after mice bearing 14-Day tumors were treated with CXCR2 or CXCR4 antagonists or both for 3 weeks. All results are presented as the mean ± SE.
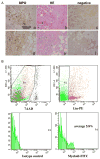
Figure 7. Immature myeloid cells in human breast tumor tissues
A: IHC staining of myeloperoxidase to identify bone marrow-derived immature myeloid cells at the invasive front of human breast ductal adenocarcinomas (a & d). H&E staining of sequential tumor sections figure b to a, and e to d, respectively. Figure c and f are negative controls. Scale bar, 100 uM for all figures. B: Flow cytometry analysis of immature myeloid cells from single cell suspension of human breast ductal adenocarcinomas (stage 2–3). The analyzed cells were gated as 7AAD negative (top left panel, P1), negative for lineage markers including CD3 for T cell, CD19 for B cell, CD56 for NK cell, CD40/CD86/HLA-DR for dendritic cells, and CD14 for monocytes, and CD33, CD34 and CD15 positive (top right panel, P2). A histogram for FITC positive myeloid cells is shown in the lower right pane, with isotype control in the lower left panel.
Figure 8. Mechanisms for enhanced tumor progression resulting from loss of TGFβ signaling
Deletion of the type II TGFβ receptor gene in mammary carcinomas results in elevated production of CXCL5, which interact with CXCR2 on Gr-1+CD11b+ cells thus recruiting the cells into the tumor microenvironment. Gr-1+CD11b+ cells, as hemaetopoietic cells, express high level of CXCR4 that interact with SDF-1 in the tumor microenvironment, and are also recruited. Gr-1+CD11b+ cells promote tumor invasion through high expression of MMP14, MMP13 and MMP2. In addition, Gr-1+CD11b+ cells produce high levels of TGFβ1 that inhibit host immune surveillance.
Similar articles
- TGFβ signaling in myeloid cells regulates mammary carcinoma cell invasion through fibroblast interactions.
Shaw AK, Pickup MW, Chytil A, Aakre M, Owens P, Moses HL, Novitskiy SV. Shaw AK, et al. PLoS One. 2015 Jan 28;10(1):e0117908. doi: 10.1371/journal.pone.0117908. eCollection 2015. PLoS One. 2015. PMID: 25629162 Free PMC article. - Deletion of TGF-β signaling in myeloid cells enhances their anti-tumorigenic properties.
Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, Moses HL. Novitskiy SV, et al. J Leukoc Biol. 2012 Sep;92(3):641-51. doi: 10.1189/jlb.1211639. Epub 2012 Jun 8. J Leukoc Biol. 2012. PMID: 22685318 Free PMC article. - Transforming growth factor-beta signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion.
Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Cheng N, et al. Mol Cancer Res. 2008 Oct;6(10):1521-33. doi: 10.1158/1541-7786.MCR-07-2203. Mol Cancer Res. 2008. PMID: 18922968 Free PMC article. - Transforming growth factor beta (TGF-beta) and inflammation in cancer.
Bierie B, Moses HL. Bierie B, et al. Cytokine Growth Factor Rev. 2010 Feb;21(1):49-59. doi: 10.1016/j.cytogfr.2009.11.008. Epub 2009 Dec 16. Cytokine Growth Factor Rev. 2010. PMID: 20018551 Free PMC article. Review. - Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer?
Yang L, Moses HL. Yang L, et al. Cancer Res. 2008 Nov 15;68(22):9107-11. doi: 10.1158/0008-5472.CAN-08-2556. Cancer Res. 2008. PMID: 19010878 Free PMC article. Review.
Cited by
- Immunomodulatory Effects of TGF-β Family Signaling within Intestinal Epithelial Cells and Carcinomas.
Smith PM, Means AL, Beauchamp RD. Smith PM, et al. Gastrointest Disord (Basel). 2019 Jun;1(2):290-300. doi: 10.3390/gidisord1020024. Epub 2019 Jun 25. Gastrointest Disord (Basel). 2019. PMID: 33834163 Free PMC article. - CD33⁺/p-STAT1⁺ double-positive cell as a prognostic factor for stage IIIa gastric cancer.
Dong J, Li J, Liu SM, Feng XY, Chen S, Chen YB, Zhang XS. Dong J, et al. Med Oncol. 2013 Mar;30(1):442. doi: 10.1007/s12032-012-0442-2. Epub 2013 Jan 11. Med Oncol. 2013. PMID: 23307253 Free PMC article. - The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis.
Itatani Y, Kawada K, Inamoto S, Yamamoto T, Ogawa R, Taketo MM, Sakai Y. Itatani Y, et al. Int J Mol Sci. 2016 Apr 28;17(5):643. doi: 10.3390/ijms17050643. Int J Mol Sci. 2016. PMID: 27136535 Free PMC article. Review. - Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models.
Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N, Vastolo V, Navas L, Garrone B, Mangano G, Biondi G, Guglielmotti A. Zollo M, et al. Clin Exp Metastasis. 2012 Aug;29(6):585-601. doi: 10.1007/s10585-012-9473-5. Epub 2012 Apr 7. Clin Exp Metastasis. 2012. PMID: 22484917
References
- Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271:20545–20550. - PubMed
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. - PubMed
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. - PubMed
- Auten RL, Richardson RM, White JR, Mason SN, Vozzelli MA, Whorton MH. Nonpeptide CXCR2 antagonist prevents neutrophil accumulation in hyperoxia-exposed newborn rats. J Pharmacol Exp Ther. 2001;299:90–95. - PubMed
- Bagadi SA, Prasad CP, Srivastava A, Prashad R, Gupta SD, Ralhan R. Frequent loss of Dab2 protein and infrequent promoter hypermethylation in breast cancer. Breast Cancer Res Treat 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA108856/CA/NCI NIH HHS/United States
- IK6 BX005225/BX/BLRD VA/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- 5P50CA098131/CA/NCI NIH HHS/United States
- CA085492/CA/NCI NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- U54 CA126505/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA116021/CA/NCI NIH HHS/United States
- CA108856/CA/NCI NIH HHS/United States
- R01 CA085492/CA/NCI NIH HHS/United States
- CA102162/CA/NCI NIH HHS/United States
- R01 CA102162/CA/NCI NIH HHS/United States
- R01 CA034590/CA/NCI NIH HHS/United States
- R01 CA076321/CA/NCI NIH HHS/United States
- CA126505/CA/NCI NIH HHS/United States
- CA068485/CA/NCI NIH HHS/United States
- P30 DK58404/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials