NCX-4016, a nitro-derivative of aspirin, inhibits EGFR and STAT3 signaling and modulates Bcl-2 proteins in cisplatin-resistant human ovarian cancer cells and xenografts - PubMed (original) (raw)
Comparative Study
NCX-4016, a nitro-derivative of aspirin, inhibits EGFR and STAT3 signaling and modulates Bcl-2 proteins in cisplatin-resistant human ovarian cancer cells and xenografts
Karuppaiyah Selvendiran et al. Cell Cycle. 2008.
Abstract
We have previously reported the inhibitory effect of NCX-4016, a nitro derivative of aspirin, on the proliferation of cisplatin-resistant human ovarian cancer cells, in vitro (Bratasz et al., Proc Natl Acad Sci USA 2006; 103:3914-9). In this report we present the results of our study on the mechanistic aspects of drug action including the molecular and signaling pathways involved in an in vitro cell line, as well as in a murine tumor xenograft. We report, for the first time, that NCX-4016 significantly inhibited the growth of cisplatin-resistant human ovarian cancer xenografts in mice. We observed that the inhibitory effect of NCX-4016 on cell proliferation was associated with G(1) phase cell cycle arrest with increased activity of p53, p21 and p27 proteins. NCX-4016 modulated the Bcl-2 family of proteins, and induced apoptosis by activating Bax and cytochrome c release in a time-dependent manner. In addition, NCX-4016 selectively down-regulated the phosphorylated forms of EGFR (Tyr845, Tyr992), pAkt (Ser473, Thr305), and STAT3 (Tyr705, Ser727), in vitro and in vivo. Taken together, the results clearly suggested that NCX-4016 causes significant induction of cell cycle arrest and apoptosis in cisplatin-resistant human ovarian cancer cells via down-regulation of EGFR/PI3K/STAT3 signaling and modulation of Bcl-2 family proteins. Thus, NCX-4016 appears to be a potential therapeutic agent for treating recurrent human ovarian carcinoma.
Figures
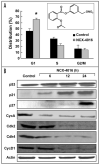
Figure 1
Effect of NCX-4016 on cell cycle distribution and cell cycle-related protein expression in cisplatin-resistant human ovarian cancer cells in vitro. (A) Cell cycle distribution. Cells were treated with either DMSO (vehicle, Control) or NCX-4016 (100 μM) for 24 h and then subjected to flow cytometric analysis. *significantly different (p < 0.05) compared to Control G1 population. The molecular structure of NCX-4016 is shown in the inset. (B) Immunoblot images of cell cycle regulatory molecules p53, p21, p27, CycA, CycD1, cdk2, and cdk4 in the CR cells treated with NCX-4016 (100 μM) for 6, 12 or 24 h. The results show significant G1 arrest and alterations in the G1 cell cycle-related protein expressions in the CR cells treated with NCX-4016.
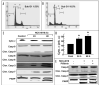
Figure 2
NCX-4016-induced apoptosis in CR ovarian cancer cells. Cells were treated with NCX-4016 (100 μM) for 48 h and the sub-G1 population for 20,000 events within a fixed gate was analyzed. Representative flow-cytometry profile for (A) control (vehicle-treated) and (B) NCX-4016-treated groups. (C) Immunoblot analysis of apoptotic proteins. Cleavages in caspase-9, caspase-8, caspase-3, caspase-7, cytochorome c, and PARP are shown in cells treated with NCX-4016 (100 μM) for 24 or 48 h. (D) Intensity of cytochrome c band as quantified by densitometry. (E) Inhibition of p53 by pifithrin did not reverse the NCX-4016 induced apoptosis. The CR cancer cells were pretreated with the pifithrin 20 μM for 1 h and then treated with NCX-4016 for 48 h. The cells were analyzed for cleaved caspase-9, caspases-7 and PARP by immunoblot assay.
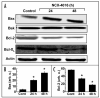
Figure 3
Effect of NCX-4016 on Bcl-2 family protein expressions in CR ovarian cancer cells. Cells were treated with NCX-4016 (100 μM) for 24 or 48 h and analyzed for various Bcl-2 family proteins. (A) Representative Western blot images showing time-dependent expressions of Bax, Bak, Bcl-2, Bcl-XL, and actin. Intensity of Bax (B) and Bcl-2 (C) as quantified by densitometry are also shown. Data represent mean ± SE of three independent experiments. *p < 0.05 as compared to the Control group. The results reveal a significant contribution of the Bcl-2 proteins, particularly Bax/Bcl-2, to the induction of apoptosis in the CR cancer cells treated with NCX-4016.
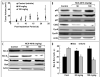
Figure 4
Effect of NCX-4016 on EGFR/PI3K and STAT3 signaling in CR ovarian cancer cells. Representative immunoblot images showing the expression levels of pEGFR, pAkt and pSTAT3 and their phosphorylated counterparts are shown. The cells were treated with NCX-4016 (100 μM) for 12 or 24 h. The results show inhibition of EGFR and STAT3 activation, but no effect on MAPK pathway, in the CR cells treated with NCX-4016.
Figure 5
Effect of NCX-4016 on ovarian cancer xenograft tumor in mice. (A) Effect of tumor volume growth in mice treated with NCX-4016. Three groups (N = 5 each) of mice were implanted with the CR ovarian cancer cells (s.c.) in the back. Five days after implantation, two groups were administered (i.p.) with 50 or 100 mg/kg NCX-4016 daily, while the control group received vehicle (DMSO) only. The results show a dose-dependent inhibition of tumor growth in the drug-treated groups, which was significantly different from the Control group (*p < 0.05) on day 18 and beyond. (B and C) show immunoblots and analysis of Bcl-2 and cell cycle-related proteins in the tissue lysates of tumor xenografts. (D) The Bcl-2 and Bax band signals were quantified by densitometry. Data represent mean ± SE of three independent experiments. *p < 0.05 as compared to the respective Control groups.
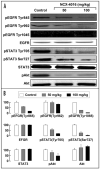
Figure 6
Effect of NCX-4016 on EGFR/PI3K and STAT3 signaling in ovarian cancer xenograft tumors in mice. (A) Immunoblot images of tumor tissue lysates, obtained as in Fig. 5 and subjected to immunoblot analysis of EGFR/ PI3K and STAT3 signaling pathways. (B) Quantification. NCX-4016, at both 50 and 100 mg/kg doses, downregulated the phosphorylated EGFR, Akt and STAT3 without significant change in their total levels.
Similar articles
- NCX-4040, a nitric oxide-releasing aspirin, sensitizes drug-resistant human ovarian xenograft tumors to cisplatin by depletion of cellular thiols.
Bratasz A, Selvendiran K, Wasowicz T, Bobko A, Khramtsov VV, Ignarro LJ, Kuppusamy P. Bratasz A, et al. J Transl Med. 2008 Feb 26;6:9. doi: 10.1186/1479-5876-6-9. J Transl Med. 2008. PMID: 18302761 Free PMC article. - HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition.
Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Rivera BK, Kálai T, Hideg K, Kuppusamy P. Selvendiran K, et al. Cancer Biol Ther. 2011 Nov 1;12(9):837-45. doi: 10.4161/cbt.12.9.17713. Epub 2011 Nov 1. Cancer Biol Ther. 2011. PMID: 21885917 - Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells.
Yue P, Zhang X, Paladino D, Sengupta B, Ahmad S, Holloway RW, Ingersoll SB, Turkson J. Yue P, et al. Oncogene. 2012 May 3;31(18):2309-22. doi: 10.1038/onc.2011.409. Epub 2011 Sep 12. Oncogene. 2012. PMID: 21909139 Free PMC article. - The p53 upregulated modulator of apoptosis (PUMA) chemosensitizes intrinsically resistant ovarian cancer cells to cisplatin by lowering the threshold set by Bcl-x(L) and Mcl-1.
Yuan Z, Cao K, Lin C, Li L, Liu HY, Zhao XY, Liu L, Deng HX, Li J, Nie CL, Wei YQ. Yuan Z, et al. Mol Med. 2011;17(11-12):1262-74. doi: 10.2119/molmed.2011.00176. Epub 2011 Aug 19. Mol Med. 2011. PMID: 21863213 Free PMC article. - Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin.
Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P. Bratasz A, et al. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3914-9. doi: 10.1073/pnas.0511250103. Epub 2006 Feb 23. Proc Natl Acad Sci U S A. 2006. PMID: 16497833 Free PMC article.
Cited by
- STAT3, the Challenge for Chemotherapeutic and Radiotherapeutic Efficacy.
Yang PL, Liu LX, Li EM, Xu LY. Yang PL, et al. Cancers (Basel). 2020 Aug 30;12(9):2459. doi: 10.3390/cancers12092459. Cancers (Basel). 2020. PMID: 32872659 Free PMC article. Review. - A novel mechanism for the anticancer activity of aspirin and salicylates.
Bashir AIJ, Kankipati CS, Jones S, Newman RM, Safrany ST, Perry CJ, Nicholl ID. Bashir AIJ, et al. Int J Oncol. 2019 Apr;54(4):1256-1270. doi: 10.3892/ijo.2019.4701. Epub 2019 Jan 29. Int J Oncol. 2019. PMID: 30720135 Free PMC article. - A natural product-like JAK2/STAT3 inhibitor induces apoptosis of malignant melanoma cells.
Wu KJ, Huang JM, Zhong HJ, Dong ZZ, Vellaisamy K, Lu JJ, Chen XP, Chiu P, Kwong DWJ, Han QB, Ma DL, Leung CH. Wu KJ, et al. PLoS One. 2017 Jun 1;12(6):e0177123. doi: 10.1371/journal.pone.0177123. eCollection 2017. PLoS One. 2017. PMID: 28570563 Free PMC article. - NCX-4040, a nitric oxide-releasing aspirin, sensitizes drug-resistant human ovarian xenograft tumors to cisplatin by depletion of cellular thiols.
Bratasz A, Selvendiran K, Wasowicz T, Bobko A, Khramtsov VV, Ignarro LJ, Kuppusamy P. Bratasz A, et al. J Transl Med. 2008 Feb 26;6:9. doi: 10.1186/1479-5876-6-9. J Transl Med. 2008. PMID: 18302761 Free PMC article. - Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3.
Selvendiran K, Bratasz A, Kuppusamy ML, Tazi MF, Rivera BK, Kuppusamy P. Selvendiran K, et al. Int J Cancer. 2009 Nov 1;125(9):2198-204. doi: 10.1002/ijc.24601. Int J Cancer. 2009. PMID: 19623660 Free PMC article.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. - PubMed
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Trope C. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. - PubMed
- Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. Eur J Surg Oncol. 2006;32:875–86. - PubMed
- Brewer CA, Blessing JA, Nagourney RA, Morgan M, Hanjani P. Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;103:446–50. - PubMed
- Pani E, Stojic L, El-Shemerly M, Jiricny J, Ferrari S. Mismatch repair status and the response of human cells to cisplatin. Cell Cycle. 2007;6:1796–802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous