IRF-1 is required for full NF-kappaB transcriptional activity at the human immunodeficiency virus type 1 long terminal repeat enhancer - PubMed (original) (raw)
. 2008 Apr;82(7):3632-41.
doi: 10.1128/JVI.00599-07. Epub 2008 Jan 23.
Anna L Remoli, Giulia Marsili, Barbara Ridolfi, Alessandra Borsetti, Edvige Perrotti, Roberto Orsatti, Ramona Ilari, Leonardo Sernicola, Emilia Stellacci, Barbara Ensoli, Angela Battistini
Affiliations
- PMID: 18216101
- PMCID: PMC2268499
- DOI: 10.1128/JVI.00599-07
IRF-1 is required for full NF-kappaB transcriptional activity at the human immunodeficiency virus type 1 long terminal repeat enhancer
Marco Sgarbanti et al. J Virol. 2008 Apr.
Abstract
Human immunodeficiency virus type 1 (HIV-1) gene expression is controlled by a complex interplay between viral and host factors. We have previously shown that interferon-regulatory factor 1 (IRF-1) is stimulated early after HIV-1 infection and regulates promoter transcriptional activity even in the absence of the viral transactivator Tat. In this work we demonstrate that IRF-1 is also required for full NF-kappaB transcriptional activity. We provide evidence that IRF-1 and NF-kappaB form a functional complex at the long terminal repeat (LTR) kappaB sites, which is abolished by specific mutations in the two adjacent kappaB sites in the enhancer region. Silencing IRF-1 with small interfering RNA resulted in impaired NF-kappaB-mediated transcriptional activity and in repressed HIV-1 transcription early in de novo-infected T cells. These data indicate that in early phases of HIV-1 infection or during virus reactivation from latency, when the viral transactivator is absent or present at very low levels, IRF-1 is an additional component of the p50/p65 heterodimer binding the LTR enhancer, absolutely required for efficient HIV-1 replication.
Figures
FIG. 1.
IRF-1 and NF-κB form an active complex on the HIV-1 enhancer region. (A) HEK293 cells were transiently cotransfected with the indicated reporter constructs (2.5 ng) corresponding to the wt or mutated LTR κB enhancer and an expression vector for IRF-1 (10 ng). Twenty-four hours after transfection, luciferase activity was measured as described in Materials and Methods. Means ± standard deviations from three separate experiments after normalization with Renilla activity are shown. RLU, relative light units. (B) Whole-cell extracts (15 μg) from IRF-1-transfected HEK293 cells were subjected to EMSA using the wt κB LTR probe or a canonical κB site. Supershift analysis was performed using specific anti-IRF-1, anti-p65, and anti-p50 antibodies. (C) Whole-cell extracts (15 μg) from IRF-1- and p50/p65-transfected HEK293 cells were subjected to EMSA using the wt κB LTR probe or probes mutated as shown in Table 1. Supershift analysis was performed as for panel B.
FIG. 2.
TNF-α-activated NF-κB associates with IRF-1 on the HIV-1 LTR enhancer, and the complex is required for full LTR enhancer transcriptional stimulation. (A) Jurkat T cells left untreated or treated with TNF-α (10 ng/ml) for 4 h. Nuclear cell extracts were immunoprecipitated (IP) with anti-IRF-1 specific antibodies, and the complexes were separated by 10% SDS-PAGE and subsequently probed with anti-IRF-1 and anti-p65 specific antibodies. Western blotting with anti-TFII H in the input is shown as a control of sample loading. IgG, immunoglobulin G. (B) Jurkat T cells left untreated or treated for the indicated time with TNF-α (10 ng/ml) and nuclear cell extracts (15 μg) incubated with oligonucleotides corresponding to HIV-1 wt κB binding sites or sites mutated as indicated in Table 1 were analyzed by EMSA (lanes 1 to 4). Supershift assays with specific antibodies against p50, p65, and IRF-1 were performed on cell extracts from cells treated with TNF-α for 15 min (lanes 5 to 8). (C) Jurkat T cells were transfected with the indicated reporter constructs (1 μg) corresponding to wt κB enhancer or enhancers mutated as for panel B, and luciferase activity was measured as described in Materials and Methods before or after stimulation with TNF-α for 9 h. Means ± standard deviations from three separate experiments after normalization with Renilla activity are shown. RLU, relative light units.
FIG. 3.
IRF-1 binds the κB enhancer in vivo and synergistically with NF-κB activates an integrated provirus. (A) 1G5 cells untreated or treated with TNF-α (10 ng/ml) for 15 min were subjected to ChIP as described in Materials and Methods, using either normal rabbit serum or anti-IRF-1 and anti-p65 antibodies. Samples were amplified by PCR, using primers specific for LTR NF-κB enhancer (lanes 1 to 8) and for the κB1 site on the IκBα gene promoter (lanes 9 to 12). (B) HLM-1 cells were transfected with an IRF-1 expression vector and treated with TNF-α (10 ng/ml). After 24 and 48 h, p24 antigen accumulation was determined in the cell supernatants as indicated in Materials and Methods. Error bars indicate standard deviations.
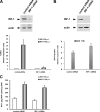
FIG. 4.
Knockdown of IRF-1 by siRNA abolishes the formation of the IRF-1/p50/p65 complex and impairs HIV-1 LTR transcriptional activity. (A) IRF-1 expression was determined in whole extracts from Jurkat T cells stably expressing either IRF-1-targeting or control siRNAs (control siRNA or Luc siRNA) by Western blotting using specific antibody against IRF-1. Western blotting using an antiactin antibody was used as control of sample loading. The intensity of the specific bands was measured by densitometry and reported as percentage of IRF-1 expression in IRF-1 siRNA-expressing cells relative to control cells after normalization with actin levels. (B) Left panel, nuclear cell extracts prepared from Jurkat T cells, stably expressing Luc siRNA (lanes 1 to 3), control siRNA (lanes 4 to 6), or IRF-1 siRNA (lanes 7 to 9) and stimulated with TNF-α for the indicated time, were subjected to EMSA using an LTR κB probe. Right panel, nuclear cell extracts from control siRNA-expressing cells stimulated for 30 min with TNF-α were further analyzed by supershift with specific antibodies against p65, p50, and IRF-1. (C) Jurkat T cells stably expressing either IRF-1 or control siRNA were transiently transfected with the wt LTR κB enhancer reporter construct (1 μg) or with constructs mutated as in Fig. 1A and stimulated with TNF-α for 9 h. Twenty-four hours after transfection, luciferase activity was measured as described in Materials and Methods. Means ± standard deviations from three separate experiments were calculated after normalization with Renilla activity. RLU, relative light units.
FIG. 5.
(A) HIV-1 transcription is inhibited by IRF-1 siRNA expression. Total RNA was purified, at different time points, from Jurkat T cells stably expressing either IRF-1 or control siRNA, infected with the HIV-1 pHXBc2 molecular clone. Primary HIV-1 transcripts were monitored by real-time RT-PCR using specific primers for early transcripts (37) Levels from uninfected cells at 5 h were set as the basis of comparative results and for GAPDH, whose sequence is indicated in Materials and Methods. (B) RT-PCR analysis was performed for SeV N protein on RNA extracts from 10-h SeV-infected control and IRF-1 siRNA-expressing Jurkat cells. Data are normalized by the level of GAPDH mRNA expression in each sample and shown as relative expression units. Western blot analysis of IRF-1 levels was performed on whole-cell extracts from control and IRF-1 siRNA-expressing Jurkat cells infected with HIV-1 (A, upper panel) or SeV (B, upper panel); actin levels are also shown as loading control. (C) Total DNA from Jurkat T cells stably expressing either IRF-1 or control siRNA and infected with HIV-1 at the indicated time points was purified, and the HIV-1 gag DNA full reverse transcript was quantified by real-time PCR as indicated in Materials and Methods. Error bars indicate standard deviations.
FIG. 6.
Schematic representation of IRF-1/NF-κB interactions on the HIV-1 LTR enhancer region. The enhancer region present in the HIV-1 LTR promoter, containing two adjacent high-affinity binding sites for NF-κB that are critical for LTR promoter activity and important for optimal HIV-1 replication, is shown. A complex between IRF-1 and NF-κB is formed upon HIV-1 infection and TNF-α treatment. Mutations in either the distal or proximal κB site prevented the formation of the IRF-1/NF-κB complex and resulted in decreased LTR transcriptional activity. A hierarchy of the two κB binding sites in terms of NF-κB binding ability is also indicated: mutations in the proximal κB site still allowed the formation of the double p50/p65 NF-κB heterodimer, whereas when the distal κB site was mutated, only a single p50/p65 heterodimer bound.
Similar articles
- Semen Exosomes Promote Transcriptional Silencing of HIV-1 by Disrupting NF-κB/Sp1/Tat Circuitry.
Welch JL, Kaddour H, Schlievert PM, Stapleton JT, Okeoma CM. Welch JL, et al. J Virol. 2018 Oct 12;92(21):e00731-18. doi: 10.1128/JVI.00731-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111566 Free PMC article. - Modulation of human immunodeficiency virus 1 replication by interferon regulatory factors.
Sgarbanti M, Borsetti A, Moscufo N, Bellocchi MC, Ridolfi B, Nappi F, Marsili G, Marziali G, Coccia EM, Ensoli B, Battistini A. Sgarbanti M, et al. J Exp Med. 2002 May 20;195(10):1359-70. doi: 10.1084/jem.20010753. J Exp Med. 2002. PMID: 12021315 Free PMC article. - Permanent occupancy of the human immunodeficiency virus type 1 enhancer by NF-kappa B is needed for persistent viral replication in monocytes.
Jacqué JM, Fernández B, Arenzana-Seisdedos F, Thomas D, Baleux F, Virelizier JL, Bachelerie F. Jacqué JM, et al. J Virol. 1996 May;70(5):2930-8. doi: 10.1128/JVI.70.5.2930-2938.1996. J Virol. 1996. PMID: 8627768 Free PMC article. - Human immunodeficiency virus type-1 transcription: role of the 5'-untranslated leader region (review).
Al-Harthi L, Roebuck KA. Al-Harthi L, et al. Int J Mol Med. 1998 May;1(5):875-81. doi: 10.3892/ijmm.1.5.875. Int J Mol Med. 1998. PMID: 9852310 Review. - Efficient Non-Epigenetic Activation of HIV Latency through the T-Cell Receptor Signalosome.
Hokello J, Sharma AL, Tyagi M. Hokello J, et al. Viruses. 2020 Aug 8;12(8):868. doi: 10.3390/v12080868. Viruses. 2020. PMID: 32784426 Free PMC article. Review.
Cited by
- IRF1 suppresses Ki-67 promoter activity through interfering with Sp1 activation.
Chen F, Song J, Di J, Zhang Q, Tian H, Zheng J. Chen F, et al. Tumour Biol. 2012 Dec;33(6):2217-25. doi: 10.1007/s13277-012-0483-3. Epub 2012 Aug 14. Tumour Biol. 2012. PMID: 22890831 - HIV-1 latency: an update of molecular mechanisms and therapeutic strategies.
Battistini A, Sgarbanti M. Battistini A, et al. Viruses. 2014 Apr 14;6(4):1715-58. doi: 10.3390/v6041715. Viruses. 2014. PMID: 24736215 Free PMC article. Review. - Early IFN type I response: Learning from microbial evasion strategies.
Coccia EM, Battistini A. Coccia EM, et al. Semin Immunol. 2015 Mar;27(2):85-101. doi: 10.1016/j.smim.2015.03.005. Epub 2015 Apr 11. Semin Immunol. 2015. PMID: 25869307 Free PMC article. Review. - Diverse therapeutic efficacies and more diverse mechanisms of nicotinamide.
Song SB, Park JS, Chung GJ, Lee IH, Hwang ES. Song SB, et al. Metabolomics. 2019 Oct 5;15(10):137. doi: 10.1007/s11306-019-1604-4. Metabolomics. 2019. PMID: 31587111 Review.
References
- Aguilar-Cordova, E., J. Chinen, L. Donehower, D. E. Lewis, and J. W. Belmont. 1994. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res. Hum Retroviruses 10295-301. - PubMed
- Alcami, J., T. Lain de Lera, L. Folgueira, M. A. Pedraza, J. M. Jacque, F. Bachelerie, A. R. Noriega, R. T. Hay, D. Harrich, R. B. Gaynor, et al. 1995. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 141552-1560. - PMC - PubMed
- Bachelerie, F., J. Alcami, F. Arenzana-Seisdedos, and J. L. Virelizier. 1991. HIV enhancer activity perpetuated by NF-kappa B induction on infection of monocytes. Nature 350709-712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials