Lithium delays progression of amyotrophic lateral sclerosis - PubMed (original) (raw)
. 2008 Feb 12;105(6):2052-7.
doi: 10.1073/pnas.0708022105. Epub 2008 Feb 4.
Patrizia Longone, Luisa Cafaro, Olga Kastsiuchenka, Michela Ferrucci, Maria Laura Manca, Gloria Lazzeri, Alida Spalloni, Natascia Bellio, Paola Lenzi, Nicola Modugno, Gabriele Siciliano, Ciro Isidoro, Luigi Murri, Stefano Ruggieri, Antonio Paparelli
Affiliations
- PMID: 18250315
- PMCID: PMC2538879
- DOI: 10.1073/pnas.0708022105
Lithium delays progression of amyotrophic lateral sclerosis
Francesco Fornai et al. Proc Natl Acad Sci U S A. 2008.
Erratum in
- Proc Natl Acad Sci U S A. 2008 Oct 21;105(42):16404-7
Abstract
ALS is a devastating neurodegenerative disorder with no effective treatment. In the present study, we found that daily doses of lithium, leading to plasma levels ranging from 0.4 to 0.8 mEq/liter, delay disease progression in human patients affected by ALS. None of the patients treated with lithium died during the 15 months of the follow-up, and disease progression was markedly attenuated when compared with age-, disease duration-, and sex-matched control patients treated with riluzole for the same amount of time. In a parallel study on a genetic ALS animal model, the G93A mouse, we found a marked neuroprotection by lithium, which delayed disease onset and duration and augmented the life span. These effects were concomitant with activation of autophagy and an increase in the number of the mitochondria in motor neurons and suppressed reactive astrogliosis. Again, lithium reduced the slow necrosis characterized by mitochondrial vacuolization and increased the number of neurons counted in lamina VII that were severely affected in saline-treated G93A mice. After lithium administration in G93A mice, the number of these neurons was higher even when compared with saline-treated WT. All these mechanisms may contribute to the effects of lithium, and these results offer a promising perspective for the treatment of human patients affected by ALS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Effects of lithium treatment on the lifespan and neurological symptoms of G93A mice. (a) Survival curve for saline- and lithium-treated G93A mice. Lithium carbonate (1 mEq/kg, daily, i.p.) treatment significantly increased the survival time of G93A mice compared with saline-treated mice. (b) Effects of lithium on specific symptoms, such as hind limb adduction, gait impairment, and the onset of severe paralysis. (c) Symptomatic effects and prolongation of the life span induced by lithium. Values represent the mean ± SEM of 10 mice per group in two different experiments (total N per group = 20). Comparison was made by using ANOVA with Sheffe's post hoc analysis. *, P ≤ 0.05 compared with G93A mice administered saline. **, P ≤ 0.001 compared with G93A mice administered saline.
Fig. 2.
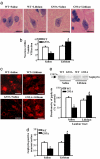
Neuroprotective effects of lithium on medium-size lamina VII neurons. (a) Shows representative micrographs of those H&E-stained lamina VII neurons that were selected for the count based on size specificity (diameter ranging from 10 to 20 μm). (b) Graph indicates the severe loss of these neurons in G93A mice, which exceeded the loss of MN. Remarkably, the G93A mice treated with lithium showed a much higher number of lamina VII medium-size neurons even compared with saline-treated WT mice. (c–e) These results were confirmed by gephyrin immunostaining, as shown here and by all of the staining procedures summarized in
SI Fig. 16
. Counts represent the mean ± SEM of 62,000 cells per group (3,100 per mouse in groups of 20 mice). Comparison among groups was made by using one-way ANOVA. *, P ≤ 0.05 compared with WT saline-treated group. #, P ≤ 0.001 compared with G93A saline-treated groups. (Scale bars, 17 μm.)
Fig. 3.
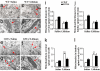
Effects of lithium administration on motor neurons mitochondria in vivo. (a–h) Representative pictures of mitochondria (arrows) in MN from the spinal cord of WT mice treated with saline (a and b) or lithium (c and d) and from G93A mice treated with saline (e and g) or lithium (f and h). (g) In G93A mice treated with saline, TEM shows mitochondrial vacuolization (arrowheads). (f and h) This vacuolization is consistently absent in mitochondria of G93A mice treated with lithium. (d and h–j) Lithium decreases the size of mitochondria both in WT and G93A mice (d and h, respectively) both in the cervical (i) and lumbar (j) spinal cord. (k and l) Lithium increases the number of mitochondria both in cervical (k) and lumbar (l) MN both in WT and G93A. Values are the mean ± SEM. Comparison between groups was made by using one-way ANOVA. *, P ≤ 0.05 compared with saline-treated mice. **, P ≤ 0.01 compared with saline treated mice. (Scale bars: a, c, e, and g, 1.8 μm; b, d, f, and h, 0.25 μm.)
Fig. 4.
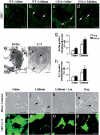
Effects of lithium on autophagy in vivo and in vitro. (a–d) MDC-positive small vacuoles in the lumbar spinal cord of WT (a and c, arrows) and G93A mice (b and d, arrows). (e) Representative picture of beclin immunostained vacuoles in the cytoplasm of alpha MN from a G93A lithium-treated mouse. The ImmunoGold particles (20 nm) are localized on both the membrane (that surround the core) and the electrondense core (arrows). (f) LC3 immunostaining is present on a larger membranous structure; the ImmunoGold particles (20 nm) are randomly localized (arrows). (g) The count of beclin immunostained structures shows a marked effect of lithium in MN both WT and G93A mice. (h) Likewise, LC3 immunopositive vacuoles increase significantly in G93A and WT mice administered lithium. (i–l) Phase-contrast microscopic images of lithium-induced accumulation of vacuoles in SH-SY5Y cells exposed or not for 72 h to 1 mM lithium (j), or lithium plus 50 mM asparagine (Asn) (a slight autophagy blocker acting downstream of lithium) (k), or 400 nM rapamycin (Rap) (l). Arrows point to cytoplasmic vacuoles that accumulate in cells treated with lithium or Rap (a known autophagy inducer). No vacuolization was observed in control (i) or lithium plus Asn-treated cells. (m–p) A parallel experiment was performed with transfected SH-SY5Y cells stably expressing the GFP-LC3 chimeric fluorescent protein. The images clearly show that both lithium (n) and rapamycin (p) change the cytoplasmic diffuse fluorescence pattern of GFP-LC3 to a punctuated pattern indicative of autophagosome formation. Asparagine (o) inhibited the effect of lithium on GFP-LC3 localization. *, P < 0.05 compared with saline. (Scale bars: a–d, 14 μm; e, 0.1 μm; f, 0.08 μm; i–l, 20 μm; m–p, 50 μm.)
Fig. 5.
Effects of lithium treatment on disease symptom progression and survival in patients with ALS. (a, b, d, and e) Symptoms progression (evaluated every 3 months) in controls (riluzole-treated) and treated patients (lithium plus riluzole-treated patients) expressed as raw data, using the Norris (a) and ALSFRS-R (b) rating scales and FVC (d) and MRC (e). There was no significant progression in the lithium-treated patients at any time interval apart from the last two evaluations using the MRC scale. In the riluzole-treated patients, symptoms progressed significantly starting at 3 or 6 months (depending on the scale). (c) Survival curve as normalized data shows the percentage of patients surviving over the 15 months of treatment in the riluzole and lithium groups. None of the patients died in the lithium-treated group; however, in the control group, although the patients had comparable disease severity at enrollment, ≈30% died. Intra- and intergroup analyses were performed by unpaired t test or ANOVA followed by the Bonferroni post hoc test. (f) Shows the breakdown between treated and control patients for FVC. (g) Shows the breakdown between groups for FVC (calculated at 1 month before death) in those patients affected by the bulbar form. *, P < 0.05, **, P < 0.01 compared with baseline value. #, P < 0.05; ##, P < 0.01 compared with control patients.
Comment in
- Lithium may slow progression of amyotrophic lateral sclerosis, but further study is needed.
Bedlack RS, Maragakis N, Heiman-Patterson T. Bedlack RS, et al. Proc Natl Acad Sci U S A. 2008 Apr 22;105(16):E17; author reply E18. doi: 10.1073/pnas.0801762105. Epub 2008 Apr 16. Proc Natl Acad Sci U S A. 2008. PMID: 18417448 Free PMC article. No abstract available. - A clinical specification for a randomized clinical trial on lithium in amyotrophic lateral sclerosis.
Vanacore N, Galeotti F. Vanacore N, et al. Proc Natl Acad Sci U S A. 2008 Jun 24;105(25):E35; author reply E36. doi: 10.1073/pnas.0801837105. Epub 2008 Jun 12. Proc Natl Acad Sci U S A. 2008. PMID: 18550833 Free PMC article. No abstract available.
Similar articles
- Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.
Fellner A, Barhum Y, Angel A, Perets N, Steiner I, Offen D, Lev N. Fellner A, et al. Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article. - An anthocyanin-enriched extract from strawberries delays disease onset and extends survival in the hSOD1G93A mouse model of amyotrophic lateral sclerosis.
Winter AN, Ross EK, Wilkins HM, Stankiewicz TR, Wallace T, Miller K, Linseman DA. Winter AN, et al. Nutr Neurosci. 2018 Jul;21(6):414-426. doi: 10.1080/1028415X.2017.1297023. Epub 2017 Mar 9. Nutr Neurosci. 2018. PMID: 28276271 - Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis.
Kim J, Kim TY, Cho KS, Kim HN, Koh JY. Kim J, et al. Neurobiol Dis. 2013 Nov;59:80-5. doi: 10.1016/j.nbd.2013.07.011. Epub 2013 Jul 26. Neurobiol Dis. 2013. PMID: 23891729 - Autophagy, lithium, and amyotrophic lateral sclerosis.
Pasquali L, Longone P, Isidoro C, Ruggieri S, Paparelli A, Fornai F. Pasquali L, et al. Muscle Nerve. 2009 Aug;40(2):173-94. doi: 10.1002/mus.21423. Muscle Nerve. 2009. PMID: 19609902 Review. - Lithium for treatment of amyotrophic lateral sclerosis: much ado about nothing.
Gamez J, Salvado M, Martínez de la Ossa A, Badia M. Gamez J, et al. Neurologia. 2016 Oct;31(8):550-61. doi: 10.1016/j.nrl.2013.02.001. Epub 2013 Apr 10. Neurologia. 2016. PMID: 23582371 Review. English, Spanish.
Cited by
- SETX (senataxin), the helicase mutated in AOA2 and ALS4, functions in autophagy regulation.
Richard P, Feng S, Tsai YL, Li W, Rinchetti P, Muhith U, Irizarry-Cole J, Stolz K, Sanz LA, Hartono S, Hoque M, Tadesse S, Seitz H, Lotti F, Hirano M, Chédin F, Tian B, Manley JL. Richard P, et al. Autophagy. 2021 Aug;17(8):1889-1906. doi: 10.1080/15548627.2020.1796292. Epub 2020 Aug 7. Autophagy. 2021. PMID: 32686621 Free PMC article. - Autophagy and ALS: mechanistic insights and therapeutic implications.
Chua JP, De Calbiac H, Kabashi E, Barmada SJ. Chua JP, et al. Autophagy. 2022 Feb;18(2):254-282. doi: 10.1080/15548627.2021.1926656. Epub 2021 May 31. Autophagy. 2022. PMID: 34057020 Free PMC article. Review. - UNC13A in amyotrophic lateral sclerosis: from genetic association to therapeutic target.
Willemse SW, Harley P, van Eijk RPA, Demaegd KC, Zelina P, Pasterkamp RJ, van Damme P, Ingre C, van Rheenen W, Veldink JH, Kiernan MC, Al-Chalabi A, van den Berg LH, Fratta P, van Es MA. Willemse SW, et al. J Neurol Neurosurg Psychiatry. 2023 Aug;94(8):649-656. doi: 10.1136/jnnp-2022-330504. Epub 2023 Feb 3. J Neurol Neurosurg Psychiatry. 2023. PMID: 36737245 Free PMC article. Review. - Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing.
Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, Pastores GM, Rubinsztein DC, Nixon RA, Duchen MR, Mallucci GR, Kroemer G, Levine B, Eskelinen EL, Mochel F, Spedding M, Louis C, Martin OR, Millan MJ. Boland B, et al. Nat Rev Drug Discov. 2018 Sep;17(9):660-688. doi: 10.1038/nrd.2018.109. Epub 2018 Aug 17. Nat Rev Drug Discov. 2018. PMID: 30116051 Free PMC article. Review. - Chemical Screening Approaches Enabling Drug Discovery of Autophagy Modulators for Biomedical Applications in Human Diseases.
Panda PK, Fahrner A, Vats S, Seranova E, Sharma V, Chipara M, Desai P, Torresi J, Rosenstock T, Kumar D, Sarkar S. Panda PK, et al. Front Cell Dev Biol. 2019 Mar 19;7:38. doi: 10.3389/fcell.2019.00038. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 30949479 Free PMC article. Review.
References
- Bruijn LI, Miller TM, Cleveland DW. Annu Rev Neurosci. 2004;27:723–749. - PubMed
- Rosen DR. Nature. 1993;362:59–62. - PubMed
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Science. 1994;264:1772–1775. - PubMed
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. Neuron. 1995;14:1105–1116. - PubMed
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Science. 2003;302:113–117. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous