Quantum model of catalysis based on a mobile proton revealed by subatomic x-ray and neutron diffraction studies of h-aldose reductase - PubMed (original) (raw)
. 2008 Feb 12;105(6):1844-8.
doi: 10.1073/pnas.0711659105. Epub 2008 Feb 4.
Federico Ruiz, Raul Cachau, Isabelle Hazemann, Flora Meilleur, Andre Mitschler, Stephan Ginell, Pavel Afonine, Oscar N Ventura, Alexandra Cousido-Siah, Michael Haertlein, Andrzej Joachimiak, Dean Myles, Alberto Podjarny
Affiliations
- PMID: 18250329
- PMCID: PMC2538850
- DOI: 10.1073/pnas.0711659105
Quantum model of catalysis based on a mobile proton revealed by subatomic x-ray and neutron diffraction studies of h-aldose reductase
Matthew P Blakeley et al. Proc Natl Acad Sci U S A. 2008.
Abstract
We present results of combined studies of the enzyme human aldose reductase (h-AR, 36 kDa) using single-crystal x-ray data (0.66 A, 100K; 0.80 A, 15K; 1.75 A, 293K), neutron Laue data (2.2 A, 293K), and quantum mechanical modeling. These complementary techniques unveil the internal organization and mobility of the hydrogen bond network that defines the properties of the catalytic engine, explaining how this promiscuous enzyme overcomes the simultaneous requirements of efficiency and promiscuity offering a general mechanistic view for this class of enzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
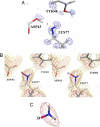
Fig. 1.
X-ray results. (A) Ribbon drawing of hydrogenated (S1) (red) and deuterated (S2) (blue) structures (rmsd = 0.7 Å), superposed with x-ray 2_F_o−_F_c density map (S2) structure, 1.5 rms magenta contours for the inhibitor IDD594. (B) Active-site conformation showing the residues around NADP+ and inhibitor IDD594 for the deuterated structure (S2). (C) Closeup of Lys-77 and Asp-43 in deuterated h-AR superposed with the two difference maps [deuterated (S2), 2 rms blue contours; and hydrogenated (S1), 2 rms red contours]. These maps show the hydrogen atom in the H-bond Asp-43–Lys-77 in two alternative conformations. This shared partial protonation is indicated also by the bond lengths (corresponding to structure S2) in the carboxylate of Asp-43. The H-atom is placed on the Lys-77 side.
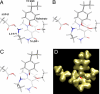
Fig. 2.
Comparison of x-ray and neutron results. (A) X-ray model of fully deuterated h-AR-IDD594 complex [0.80-Å data collected at 15K, S2; treatment with HKL2000 (16); refinement with SHELX (17)] superposed with electron density difference map (_F_o−_F_c, 2 rms blue contours, phases calculated from model without deuteriums). The map suggests partial deuteration of the Asp-43. The model shows the neutral state for the Asp-43-Lys-77 pair. (B) Model from joint x-ray/neutron refinement of fully deuterated h-AR-IDD594 complex (S3) (both data collected at room temperature) superposed with neutron scattering-density map calculated with phases from the model with all deuteriums (2_F_o−_F_c, 2 rms red contours, 1 rms gold contours, S3). Note that the deuterium atom D (marked in magenta) was included in the model but is only weakly present in the map, confirming the partial protonation of the Asp-43-Lys-77 pair. (C) Closeup of B centered on the Lys-77 head. The map (2 rms red contours) shows strong density for only the two deuteriums marked in light gray.
Fig. 3.
QM/MD results. (A–C) Three steps of the QM/MD calculations before (A), during (B), and after (C) PT. The calculations suggest that the presence of the charged nicotinamide head has a marked influence in the behavior of the reaction centre region. The initial hydride transfer step provokes a driving force for PT, because of both the charge on the substrate and the effect on the Lys component of the salt bridge, which donates back a proton to Asp to reduce its positive charge, thus triggering the movement of Tyr and the consequent PT. Note the shortening of the distance between Lys 77 and Tyr-48 between frames A and B. (D) Map corresponding to PT step (B) computed using quantum chemical methods. The image shows the important residues in the pathway, especially Asp-43, Lys-77, and Tyr-48, as well as the substrate. Note the density (red arrow) around the oxygen atom of Tyr-48 extending over the Lys-77 nitrogen, in good agreement with the experiment.
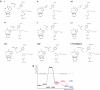
Fig. 4.
Proposed reaction pathway. (A) I–VI show the proposed reaction pathway. VII and VIII show the proton donation for a “charged” model, in which both Lys-77 and Asp-43 are charged. VI* shows the complex with the inhibitor IDD 594. (B) Energies calculated for each reaction step. The proposed pathway I–VI is shown in black (before hydride donation) and blue (after hydride donation) lines. The “charged model” pathway is included (red lines) after hydride donation (VII–VIII–VI). Note that for the proposed model, the proton donation is barrierless (blue lines), whereas for the “charged model,” there is an energy barrier (red lines).
Similar articles
- High-resolution neutron protein crystallography with radically small crystal volumes: application of perdeuteration to human aldose reductase.
Hazemann I, Dauvergne MT, Blakeley MP, Meilleur F, Haertlein M, Van Dorsselaer A, Mitschler A, Myles DA, Podjarny A. Hazemann I, et al. Acta Crystallogr D Biol Crystallogr. 2005 Oct;61(Pt 10):1413-7. doi: 10.1107/S0907444905024285. Epub 2005 Sep 28. Acta Crystallogr D Biol Crystallogr. 2005. PMID: 16204895 - Comparison of hydrogen determination with X-ray and neutron crystallography in a human aldose reductase-inhibitor complex.
Blakeley MP, Mitschler A, Hazemann I, Meilleur F, Myles DA, Podjarny A. Blakeley MP, et al. Eur Biophys J. 2006 Sep;35(7):577-83. doi: 10.1007/s00249-006-0064-8. Epub 2006 Apr 19. Eur Biophys J. 2006. PMID: 16622654 - Catalytic mechanism of aldose reductase studied by the combined potentials of quantum mechanics and molecular mechanics.
Lee YS, Hodoscek M, Brooks BR, Kador PF. Lee YS, et al. Biophys Chem. 1998 Mar 9;70(3):203-16. doi: 10.1016/s0301-4622(97)00115-4. Biophys Chem. 1998. PMID: 9546197 - Aldose reductase catalysis and crystallography. Insights from recent advances in enzyme structure and function.
Petrash JM, Tarle I, Wilson DK, Quiocho FA. Petrash JM, et al. Diabetes. 1994 Aug;43(8):955-9. doi: 10.2337/diab.43.8.955. Diabetes. 1994. PMID: 8039602 Review. - Large crystal growth by thermal control allows combined X-ray and neutron crystallographic studies to elucidate the protonation states in Aspergillus flavus urate oxidase.
Oksanen E, Blakeley MP, Bonneté F, Dauvergne MT, Dauvergne F, Budayova-Spano M. Oksanen E, et al. J R Soc Interface. 2009 Oct 6;6 Suppl 5(Suppl 5):S599-610. doi: 10.1098/rsif.2009.0162.focus. Epub 2009 Jul 8. J R Soc Interface. 2009. PMID: 19586953 Free PMC article. Review.
Cited by
- Improving the accuracy and resolution of neutron crystallographic data by three-dimensional profile fitting of Bragg peaks in reciprocal space.
Sullivan B, Archibald R, Langan PS, Dobbek H, Bommer M, McFeeters RL, Coates L, Wang X, Gallmeier F, Carpenter JM, Lynch V, Langan P. Sullivan B, et al. Acta Crystallogr D Struct Biol. 2018 Nov 1;74(Pt 11):1085-1095. doi: 10.1107/S2059798318013347. Epub 2018 Oct 29. Acta Crystallogr D Struct Biol. 2018. PMID: 30387767 Free PMC article. - Preliminary neutron crystallographic study of human transthyretin.
Haupt M, Blakeley MP, Teixeira SC, Mason SA, Mitchell EP, Cooper JB, Forsyth VT. Haupt M, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Nov 1;67(Pt 11):1428-31. doi: 10.1107/S1744309111036244. Epub 2011 Oct 27. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22102249 Free PMC article. - Aldose reductase inhibition alleviates diabetic cardiomyopathy and is associated with a decrease in myocardial fatty acid oxidation.
Gopal K, Karwi QG, Tabatabaei Dakhili SA, Wagg CS, Zhang L, Sun Q, Saed CT, Panidarapu S, Perfetti R, Ramasamy R, Ussher JR, Lopaschuk GD. Gopal K, et al. Cardiovasc Diabetol. 2023 Mar 28;22(1):73. doi: 10.1186/s12933-023-01811-w. Cardiovasc Diabetol. 2023. PMID: 36978133 Free PMC article. - High-throughput crystallography for structural genomics.
Joachimiak A. Joachimiak A. Curr Opin Struct Biol. 2009 Oct;19(5):573-84. doi: 10.1016/j.sbi.2009.08.002. Epub 2009 Sep 16. Curr Opin Struct Biol. 2009. PMID: 19765976 Free PMC article. Review. - Generalized X-ray and neutron crystallographic analysis: more accurate and complete structures for biological macromolecules.
Adams PD, Mustyakimov M, Afonine PV, Langan P. Adams PD, et al. Acta Crystallogr D Biol Crystallogr. 2009 Jun;65(Pt 6):567-73. doi: 10.1107/S0907444909011548. Epub 2009 May 15. Acta Crystallogr D Biol Crystallogr. 2009. PMID: 19465771 Free PMC article.
References
- Bernal JD, Fowler RH. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J Chem Phys. 1933;1:515–548.
- Howard EI, et al. Ultra-high resolution drug design I: Human aldose reductase-inhibitor complex at 0.66 Å shows experimentally protonation states and atomic interactions which have implications for the inhibition mechanism. Proteins Struct Funct Genet. 2004;55:792–804. - PubMed
- Yabe-Nishimura C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–33. - PubMed
- Wermuth B. In: Enzymology of Carbonyl Metabolism 2: Aldehyde Dehydrogenase, Aldo-Keto Reductase, and Alcohol Dehydrogenase. Flynn TG, Weiner H, editors. New York: Liss; 1985. pp. 209–230.
- Rondeau J-M, et al. Novel NADPH-binding domain revealed by the crystal structure of aldose reductase. Nature. 1992;355:469–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous