In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells - PubMed (original) (raw)
Comparative Study
In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells
Amy Hacker et al. Cancer Chemother Pharmacol. 2008 Dec.
Abstract
Purpose: Polyamines are essential for normal growth; however, the requirement for, and the metabolism of, polyamines are frequently dysregulated in cancer. Polyamine analogues have demonstrated promising preclinical results in multiple model systems of cancer, but their clinical utility has been limited by apparent toxicity. A representative compound of a new generation of short chain, conformationally restricted polyamine analogues, CGC-11047 has been synthesized and ongoing phase I clinical trials indicate it to be well tolerated at weekly doses of 610 mg (dose escalation is still in progress). Therefore, studies were designed to gain a better understanding of its effects on cellular polyamine biochemistry and efficacy in the treatment of human lung cancer models in vitro and in vivo.
Methods: Human lung cancers cell lines representing non-small cell and small cell lung cancers were investigated for their growth and biochemical response to CGC-11047. Effects of in vitro treatment with CGC-11047 on cell growth, the activity of the polyamine biosynthetic enzyme ornithine decarboxylase (ODC), and the expression and activity of the polyamine catabolic enzymes spermidine/spermine N(1)-acetyltransferase (SSAT) and spermine oxides (SMO) were measured. Additionally, the overall effects on intracellular polyamine pools were monitored. Finally, the in vivo efficacy of CGC-11047 in the treatment of a nude mouse model of human non-small cell lung cancer was evaluated.
Results: CGC-11047 effectively inhibited the growth of both small cell and non-small cell lung cancer cells in vitro. The greatest biochemical effects were observed in the non-small cell lung cancer cells where in addition to a profound down regulation of ODC activity, there was a significant increase in polyamine catabolism leading to a greater degree of polyamine pool depletion and greater accumulation of CGC-11047 when compared with the changes observed for the small cell lines. Importantly, CGC-11047 was found to be highly significant (P < 0.0001) in delaying the progression of established tumors in an in vivo model of human non-small cell lung cancer.
Conclusion: CGC-11047 represents a promising new polyamine analogue that warrants further preclinical and, potentially, clinical evaluation in lung cancer.
Figures
Fig. 1
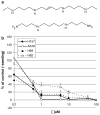
Effects of the conformationally restricted polyamine analogue, CGC-11047 of growth of human lung cancer cells in vitro. a Structures of CGC-1047 and the natural polyamine spermine. b CGC-11047 inhibits growth in human non-small cell and small cell lung cancer lines in vitro. Growth inhibition induced by increasing concentrations of CGC-11047 in the non-small cell (H157, A549) and small cell (H82, H69) lung cancer lines was determined by trypan blue exclusion cell after 96-hour treatment. Each point represents three independent experiments ±SE
Fig. 2
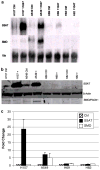
Induction of polyamine catabolic enzymes at the level of mRNA and protein. a Northern blot analysis of CGC-11047 human lung cancer cell lines. Cells were exposed to 10 μM CGC-11047 for 24 h, where indicated. Each lane was loaded with 10 μg of total RNA and blots were sequentially probed with labeled SMO and SSAT cDNAs with 18s rRNA serving as a loading control. The illustrated blot is representative of three independent experiments. b Western analysis of CGC-11047-treated human lung cancer cells. Cells were exposed to10 μM CGC-11047 for 24 h, where indicated and 15 μg of total cellular protein was loaded per lane. After transfer, membranes were sequentially incubated with SSAT, SMO, or β-actin antbodies, as indicated. The illustrated blot is representative of three independent experiments. c Quantified values of protein expression normalized to β-actin. Values represent the mean of three independent experiments (±SD) and are shown as the fold change over the control
Fig. 3
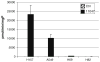
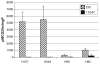
CGC-11047 induces SSAT enzyme activity in non-small cell lung cancer cell lines. Cells were treated with 10 μM CGC-11047 for 24 h and SSAT activity was determined. Striped bars represent control levels and solid bars represent treated cells. The activity values are shown in pmols/min/mg protein. Each bar represents three independent experiments performed in quadruplicate (±SE)
Fig. 4
CGC-11047 induces SMO activity in the A459 non-small cell lung cancer cell line. Cells were treated with 10 μM CGC-11047 for 24 h and assayed for SMO activity. The striped bars represent controls and solid bars represent treated cells. The activity values are shown in pmols/min/mg protein. Each bar represents three independent experiments performed in triplicate (±SE)
Fig. 5
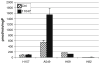
CGC-11047 treatment decreases ODC activity in human lung cancer cells. Cell lines were treated with 10 μM CGC-11047 for 24 h and ODC activity in treated a control cells was determined. The striped bars represent controls and solid bars represent treated cells. The activity values are shown in pmols CO2/hour/mg protein. Each bar represents a minimum of two independent experiments performed in quadruplicate (±SE)
Fig. 6
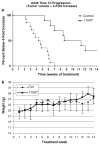
a Treatment of CGC-11047 on A549 cells in vivo increases the time to progression. Tumor cells were implanted in mice and allowed to grow until they reached a minimum of 200 mm3 prior to treatment. Animals with established tumors were then treated once a week as indicate in “Materials and Methods” with either 100 mg/kg CGC-11047 or HBSS. Tumors were measured weekly as described and the fold-increase over week one was calculated. Kaplan–Meier Analysis indicates that CGC11047-treatment significantly slows in vivo tumor growth compared to untreated controls (P ≤ 0.0001, using the Mantel–Cox log rank test). b CGC11047 does not effect normal weight gain of treated animals
Similar articles
- Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine/spermine-N1-acetyltransferase (SSAT) and as potential antitumor agents.
Saab NH, West EE, Bieszk NC, Preuss CV, Mank AR, Casero RA Jr, Woster PM. Saab NH, et al. J Med Chem. 1993 Oct 1;36(20):2998-3004. doi: 10.1021/jm00072a020. J Med Chem. 1993. PMID: 8411017 - Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells.
Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA. Devereux W, et al. Cancer Chemother Pharmacol. 2003 Nov;52(5):383-90. doi: 10.1007/s00280-003-0662-4. Epub 2003 Jun 25. Cancer Chemother Pharmacol. 2003. PMID: 12827295 - Growth and biochemical effects of unsymmetrically substituted polyamine analogues in human lung tumor cells 1.
Casero RA Jr, Mank AR, Saab NH, Wu R, Dyer WJ, Woster PM. Casero RA Jr, et al. Cancer Chemother Pharmacol. 1995;36(1):69-74. doi: 10.1007/BF00685735. Cancer Chemother Pharmacol. 1995. PMID: 7720179 - Significance of targeting polyamine metabolism as an antineoplastic strategy: unique targets for polyamine analogues.
Casero RA Jr, Frydman B, Stewart TM, Woster PM. Casero RA Jr, et al. Proc West Pharmacol Soc. 2005;48:24-30. Proc West Pharmacol Soc. 2005. PMID: 16416654 Review. - Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer.
Thomas TJ, Thomas T. Thomas TJ, et al. Med Sci (Basel). 2018 Mar 13;6(1):24. doi: 10.3390/medsci6010024. Med Sci (Basel). 2018. PMID: 29533973 Free PMC article. Review.
Cited by
- A systems analysis of the chemosensitivity of breast cancer cells to the polyamine analogue PG-11047.
Kuo WL, Das D, Ziyad S, Bhattacharya S, Gibb WJ, Heiser LM, Sadanandam A, Fontenay GV, Hu Z, Wang NJ, Bayani N, Feiler HS, Neve RM, Wyrobek AJ, Spellman PT, Marton LJ, Gray JW. Kuo WL, et al. BMC Med. 2009 Dec 14;7:77. doi: 10.1186/1741-7015-7-77. BMC Med. 2009. PMID: 20003408 Free PMC article. - Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications.
Nichugovskiy A, Tron GC, Maslov M. Nichugovskiy A, et al. Molecules. 2021 Oct 30;26(21):6579. doi: 10.3390/molecules26216579. Molecules. 2021. PMID: 34770986 Free PMC article. Review. - Recent advances in the development of polyamine analogues as antitumor agents.
Casero RA Jr, Woster PM. Casero RA Jr, et al. J Med Chem. 2009 Aug 13;52(15):4551-73. doi: 10.1021/jm900187v. J Med Chem. 2009. PMID: 19534534 Free PMC article. Review. No abstract available. - Histone deacetylase inhibition overcomes drug resistance through a miRNA-dependent mechanism.
Murray-Stewart T, Hanigan CL, Woster PM, Marton LJ, Casero RA Jr. Murray-Stewart T, et al. Mol Cancer Ther. 2013 Oct;12(10):2088-99. doi: 10.1158/1535-7163.MCT-13-0418. Epub 2013 Aug 13. Mol Cancer Ther. 2013. PMID: 23943804 Free PMC article. - Current status of the polyamine research field.
Pegg AE, Casero RA Jr. Pegg AE, et al. Methods Mol Biol. 2011;720:3-35. doi: 10.1007/978-1-61779-034-8_1. Methods Mol Biol. 2011. PMID: 21318864 Free PMC article. Review.
References
- Abeloff MD, Slavik M, Luk GD, Griffin CA, Hermann J, Blanc O, Sjoerdsma A, Baylin SB. Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. J Clin Oncol. 1984;2:124–130. - PubMed
- Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase ii trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep. 1986;70:843–845. - PubMed
- Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. - PubMed
- Bergeron RJ, McManis JS, Liu CZ, Feng Y, Weimar WR, Luchetta GR, Wu Q, Ortiz-Ocasio J, Vinson JR, Kramer D, et al. Antiproliferative properties of polyamine analogues: A structure-activity study. J Med Chem. 1994;37:3464–3476. - PubMed
- Bergeron RJ, Feng Y, Weimar WR, McManis JS, Dimova H, Porter C, Raisler B, Phanstiel O. A comparison of structure-activity relationships between spermidine and spermine analogue antineoplastics. J Med Chem. 1997;40:1475–1494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous