Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells - PubMed (original) (raw)
Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells
G-One Ahn et al. Cancer Cell. 2008 Mar.
Abstract
Tumor vasculature is derived from sprouting of local vessels (angiogenesis) and bone marrow (BM)-derived circulating cells (vasculogenesis). By using a model system of transplanting tumors into an irradiated normal tissue to prevent angiogenesis, we found that tumors were unable to grow in matrix metalloproteinase-9 (MMP-9) knockout mice, but tumor growth could be restored by transplantation of wild-type BM. Endothelial progenitor cells did not contribute significantly to this process. Rather, CD11b-positive myelomonocytic cells from the transplanted BM were responsible for tumor growth and the development of immature blood vessels in MMP-9 knockout mice receiving wild-type BM. Our results suggest that MMP-9 could be an important target for adjunct therapy to enhance the response of tumors to radiotherapy.
Figures
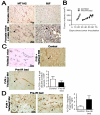
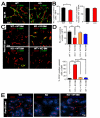
Figure 1. BM-derived EPCs contribute minimally to tumor vasculogenesis
A: MT1A2 and RIF tumors grown in female mice (upper panel) or in female mice that had received syngeneic male BM cells (lower panel). The tumors were stained for CD31 (brown) and Y-DNA probe by in situ hybridization (dark blue). Arrow heads (black) in the lower panel indicate cells positively stained for the Y-probe. B: Growth kinetics of MT1A2 tumors grown in non-irradiated (control; open symbols) or pre-irradiated (pre-IR bed; filled symbols) subcutaneous tissues of FVB mice. C: MT1A2 tumors grown in _Tie2_lacZ transgenic mice (Tie2lacZ mice) or in FVB mice that had received BM cells from _Tie2_lacZ mice (FVB + Tie2lacZ BM). Tumors in FVB + Tie2lacZ BM were grown in non-irradiated (control) or pre-irradiated (pre-IR bed) tissues. Vessels were stained with CD31 (brown) and X-gal (blue). Arrow heads (black) indicate some of the BM-derived LacZ positive cells. The graph shows quantification of CD31 and X-gal double positive vessels cells as a percentage of the total CD31 positive cells in high power field (HPF). The difference between control and pre-IR bed was not statistically significant (P > 0.05). D: A significant number of X-gal positive (blue) but CD31 (brown) negative cells were observed in the tumors grown in the pre-irradiated tissues (pre-IR bed) of FVB + Tie2lacZ BM (indicated with black arrow heads). Quantification of X-gal positive but CD31 negative cells counted per HPF is shown on the right. The data was not however significantly different between control and pre-IR bed (P > 0.05). Symbols and error bars in B, C, and D are the mean ± SEM for n ≥ 5 mice per group.
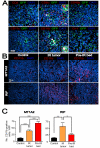
Figure 2. BM-derived CD11b+ myelomonocytic cells are recruited to the tumors with IR
A: Immunostaining of MT1A2 tumors grown in the pre-irradiated tissues of FVB mice that had received BM cells from mice ubiquitously expressing GFP. Tumor sections were stained for CD4 (red), CD8α (red), CD49b (red), CD11c (red), CD11b (red), or Gr-1 (red), and anti-GFP (green), counterstained with DAPI (blue). Co-localization of red, green, and blue is shown in white. Insets in the middle panels show magnified regions of the microphotograph where indicated with asterisks (*). B: Immunostaining for CD11b (red) in tumors with no IR (control), irradiated tumors (IR tumor), or tumors grown in the irradiated bed (pre-IR bed) for MT1A2 (upper panel) and RIF (lower panel). Nucleus staining with DAPI is shown in blue. C: Quantification of CD11b+ myelomonocytic cells in B for MT1A2 (left) and RIF (right) tumors. Symbols and error bars represent the mean ± SEM for n ≥ 4 animals per group. *, **, and *** indicates P values of < 0.05, < 0.01, and < 0.001, respectively, determined by one-way ANOVA.
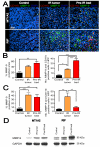
Figure 3. CD11b+ myelomonocytic cells express MMP-9 in the tumors
A: Double label immunofluorescent staining for CD11b (red) and MMP-9 (green) in MT1A2 (upper panel) and RIF (lower panel) tumors with no IR (control), irradiated tumors (IR tumor), or grown in pre-irradiated tissues (pre-IR bed). Co localization of CD11b and MMP-9 is shown in white with DAPI counterstaining. B: Quantification of MMP-9 positive area determined by the point-count method (left) and the number of CD11b and MMP-9 double positive cells in non necrotic regions (right) of MT1A2 tumors. C: RIF tumors as in B. Symbols and error bars in B and C are mean ± SEM for n ≥ 4 animals per group. *, **, and *** denote for P values < 0.05, < 0.01, < 0.001, respectively, by one-way ANOVA. D: Western blot for MMP-9 in MT1A2 (left panel) and RIF (right panel) tumors. GAPDH was used as the loading control.
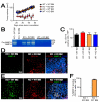
Figure 4. Functional BM cells restore tumor growth in pre-irradiated tissues of MMP-9 KO mice
A: MT1A2 tumor growth in pre-irradiated tissues of WT mice that had received the BM cells from WT mice (WT + WT BM), MMP-9 KO mice receiving BM cells from MMP-9 KO mice (KO + KO BM), MMP-9 KO mice receiving BM cells from WT mice (KO + WT BM), or WT mice that had received the BM cells from MMP-9 KO mice (WT + KO BM). Note that tumor growth was severely impaired in KO + KO BM, and was restored in KO + WT BM. B: Zymography was used to determine the efficiency of the BM reconstitution. Whole BM cells of the recipients were isolated and analyzed for MMP-9 activity at ≥ 4 weeks post BM transplantation. Each lane represents one mouse. C: Quantification of CD11b+ myelomonocytic cells in HPF from tumors in A. D: Immunostaining of the tumors from KO + KO BM for CD11b myelomonocytic cells (red) and MMP-9 (green), counterstained with DAPI (blue). E: Immunostaining of the tumors from KO + WT BM as in D. F: Quantification of MMP-9 and CD11b double positive cells in tumors from KO + KO BM or KO+ WT BM. Symbols in A, C, and F are the mean ± SEM for n ≥ 5 mice per group.
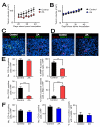
Figure 5. Immature vessels are developed in MMP-9 KO mice + WT BM to support tumor growth in pre-irradiated tissues
A: Immunostaining of CD31 (red) and α-SMA (green) in MT1A2 tumors grown in the wild-type (WT) or MMP-9 KO (KO) mice. B: Number of CD31 positive vessels per HPF (left) and proportions of CD31 positive vessels associated with α-SMA (right) in tumors from A. C: Immunostaining of CD31 (red) and α-SMA (green) for tumors from Figure 4A. Group names are as in Figure 4A. D: Quantification of CD31 positive vessels per HPF (upper panel) and proportions of CD31 positive vessels associated with α-SMA (lower panel) in the tumors from C. Symbols in B and D are the mean ± SEM for n ≥ 5 animals per group. *, **, and *** denote for _P_-values < 0.05, < 0.01, < 0.001, respectively, determined by one-way ANOVA. E: Functional blood vessels of tumors grown in pre-irradiated tissues of WT, KO, or KO + WT BM examined by intravenous infusion with Hoechst 33342 (blue). Tumor sections were stained with CD31 (red).
Figure 6. Pharmacological targeting of MMP-9 in CD11b+ myelomonocytic cells by ZA inhibits tumor growth in the pre-irradiated site
A: MT1A2 tumor growth with (ZA, n = 5) or without ZA (control, n = 4) in pre-irradiated tissues of MMP-9 KO mice that had received BM cells from WT mice. B: MT1A2 tumor growth in pre-irradiated tissues of WT mice receiving BM cells from MMP-9 KO mice. n = 5 animals per group. Symbols and error bars in A and B are the mean ± SEM. C: Immunostaining of the tumors from A for MMP-9 (green), CD11b (red), and nuclei (blue). D: Immunostaining of the tumors from B as in C. E: Quantification of CD11b+ cells (upper left), percentage of MMP-9 expressing CD11b+ cells (upper right), CD31 positive endothelial cells (lower left), and CD31 positive vessels that were associated with α-SMA (lower right) in the tumors from A. F: Quantification of the parameters as in E but from tumors in B. Error bars in E and F are SEM. ** and *** denote for _P_-values < 0.01 and < 0.001, respectively, determined by two-tailed Student’s _t_-test.
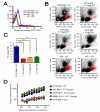
Figure 7. Depletion of CD11b+ cells expressing MMP-9 by DT further abrogates tumor growth in pre-irradiated tissues of MMP-9 KO + DTR BM
A: Peripheral blood of MMP-9 KO mice that had received BM cells from transgenic mice expressing diphtheria toxin receptor (DTR) and GFP driven by CD11b promoter (DTR BM) or of WT mice without BM transplantation (WT). These mice were treated with or without diphtheria toxin (DT, 10 ng/g). Blood was analyzed for GFP fluorescent intensity as an indicator of DTR levels at 24 hr post treatment. Note that treatment with DT 10 ng/g in DTR BM mice resulted in GFP signal similar to that in WT mice with or without DT. B: Peripheral blood of MMP-9 KO mice + DTR BM (DTR BM) treated without DT (-DT), with 10 ng/g DT, 5 ng/g DT, or 2 mg/g carrageenan (Car) at 24 hr post treatment. Blood of WT mice (WT) with and without DT 10 ng/g is also shown. Abbreviations are: R, red blood cells; L, lymphocytes; G, granulocytes; M, monocytes. C: Quantification of monocytes gated in red from B. Symbols and error bars indicate the mean ± SEM for n ≥ 5 mice per group. *** indicates P < 0.001 analyzed by one way ANOVA. D: MT1A2 tumor growth in pre-irradiated tissues of MMP-9 KO mice + DTR BM (DTR BM) without DT (−DT, n = 4), with DT (5 ng/g, n = 5), or with Car (n = 4). Tumors were also grown in pre-irradiated tissues of WT mice receiving BM cells from WT mice (WT BM, n = 5) or MMP-9 KO mice (MMP-9 KO, n = 5) that were treated with DT (5 ng/g). Error bars indicate the SEM for the number of animals indicated in the parentheses.
Comment in
- A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization.
Seandel M, Butler J, Lyden D, Rafii S. Seandel M, et al. Cancer Cell. 2008 Mar;13(3):181-3. doi: 10.1016/j.ccr.2008.02.016. Cancer Cell. 2008. PMID: 18328420 Free PMC article.
Similar articles
- A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization.
Seandel M, Butler J, Lyden D, Rafii S. Seandel M, et al. Cancer Cell. 2008 Mar;13(3):181-3. doi: 10.1016/j.ccr.2008.02.016. Cancer Cell. 2008. PMID: 18328420 Free PMC article. - Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma.
Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Melani C, et al. Cancer Res. 2007 Dec 1;67(23):11438-46. doi: 10.1158/0008-5472.CAN-07-1882. Cancer Res. 2007. PMID: 18056472 Free PMC article. - HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion.
Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. Du R, et al. Cancer Cell. 2008 Mar;13(3):206-20. doi: 10.1016/j.ccr.2008.01.034. Cancer Cell. 2008. PMID: 18328425 Free PMC article. - Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature.
Ahn GO, Brown JM. Ahn GO, et al. Angiogenesis. 2009;12(2):159-64. doi: 10.1007/s10456-009-9135-7. Epub 2009 Feb 17. Angiogenesis. 2009. PMID: 19221886 Free PMC article. Review. - Inhibiting vasculogenesis after radiation: a new paradigm to improve local control by radiotherapy.
Martin BJ. Martin BJ. Semin Radiat Oncol. 2013 Oct;23(4):281-7. doi: 10.1016/j.semradonc.2013.05.002. Semin Radiat Oncol. 2013. PMID: 24012342 Free PMC article. Review.
Cited by
- Macrophage metabolic reprogramming aggravates aortic dissection through the HIF1α-ADAM17 pathway✰.
Lian G, Li X, Zhang L, Zhang Y, Sun L, Zhang X, Liu H, Pang Y, Kong W, Zhang T, Wang X, Jiang C. Lian G, et al. EBioMedicine. 2019 Nov;49:291-304. doi: 10.1016/j.ebiom.2019.09.041. Epub 2019 Oct 19. EBioMedicine. 2019. PMID: 31640947 Free PMC article. - Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment.
Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Ahn GO, et al. Proc Natl Acad Sci U S A. 2010 May 4;107(18):8363-8. doi: 10.1073/pnas.0911378107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404138 Free PMC article. - Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting.
Imaizumi N, Monnier Y, Hegi M, Mirimanoff RO, Rüegg C. Imaizumi N, et al. PLoS One. 2010 Jun 11;5(6):e11084. doi: 10.1371/journal.pone.0011084. PLoS One. 2010. PMID: 20552031 Free PMC article. - Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy.
Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Jarosz-Biej M, et al. Int J Mol Sci. 2019 Jun 29;20(13):3212. doi: 10.3390/ijms20133212. Int J Mol Sci. 2019. PMID: 31261963 Free PMC article. Review. - Understanding tumor-stroma interplays for targeted therapies by armed mesenchymal stromal progenitors: the Mesenkillers.
Grisendi G, Bussolari R, Veronesi E, Piccinno S, Burns JS, De Santis G, Loschi P, Pignatti M, Di Benedetto F, Ballarin R, Di Gregorio C, Guarneri V, Piccinini L, Horwitz EM, Paolucci P, Conte P, Dominici M. Grisendi G, et al. Am J Cancer Res. 2011;1(6):787-805. Epub 2011 May 28. Am J Cancer Res. 2011. PMID: 22016827 Free PMC article.
References
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. - PubMed
- Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. - PubMed
- Baeten CIM, Castermans K, Lammering G, Hillen F, Wouters BG, Hillen HFP, Griffioen AW, Baeten CGM. Effects of radiotherapy and chemotherapy on angiogenesis and leukocyte infiltration in rectal cancer. Int J Rad Oncol Biol Phys. 2006;66:1219–1227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous