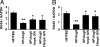PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis - PubMed (original) (raw)
. 2008 Mar 18;105(11):4277-82.
doi: 10.1073/pnas.0708647105. Epub 2008 Mar 12.
Joey Liu, Fen Yin, Alan R Collins, Christopher J Lyon, Chih-Hao Lee, Annette R Atkins, Michael Downes, Grant D Barish, Ronald M Evans, Willa A Hsueh, Rajendra K Tangirala
Affiliations
- PMID: 18337495
- PMCID: PMC2393800
- DOI: 10.1073/pnas.0708647105
PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis
Yasunori Takata et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Activation of the nuclear hormone receptor peroxisome proliferator-activated receptor delta (PPARdelta) has been shown to improve insulin resistance, adiposity, and plasma HDL levels. However, its antiatherogenic role remains controversial. Here we report atheroprotective effects of PPARdelta activation in a model of angiotensin II (AngII)-accelerated atherosclerosis, characterized by increased vascular inflammation related to repression of an antiinflammatory corepressor, B cell lymphoma-6 (Bcl-6), and the regulators of G protein-coupled signaling (RGS) proteins RGS4 and RGS5. In this model, administration of the PPARdelta agonist GW0742 (1 or 10 mg/kg) substantially attenuated AngII-accelerated atherosclerosis without altering blood pressure and increased vascular expression of Bcl-6, RGS4, and RGS5, which was associated with suppression of inflammatory and atherogenic gene expression in the artery. In vitro studies demonstrated similar changes in AngII-treated macrophages: PPARdelta activation increased both total and free Bcl-6 levels and inhibited AngII activation of MAP kinases, p38, and ERK1/2. These studies uncover crucial proinflammatory mechanisms of AngII and highlight actions of PPARdelta activation to inhibit AngII signaling, which is atheroprotective.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
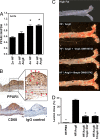
Fig. 1.
PPARδ activation attenuates AngII-accelerated atherosclerosis. Male LDLR−/− mice were treated with HF/PBS, HF/AngII, or HF/AngII/GW0742 (1 mpk or 10 mpk) for 4 weeks. (A) Aortic PPARδ mRNA expression after 2 and 4 weeks of HF/AngII treatment. Data are mean ± SD (n = 12 per group). *, P < 0.01 vs. HF. (B) Localization of aorta PPARδ protein expression by immunohistochemistry. PPARδ protein expression localized predominantly to macrophage-rich areas (CD68-positive intimal layer) and intimal vascular smooth muscle cells in lesions. (Magnification: ×40.) (C) Representative Sudan IV-stained aortas. (D) Quantification of en face atherosclerotic lesion coverage. Data are mean ± SD (n = 8 per group). *, P < 0.01 vs. HF/AngII by ANOVA.
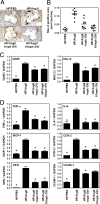
Fig. 2.
PPARδ activation suppresses AngII-induced vascular inflammation macrophage infiltration. (A) Macrophage abundance (Mac-2 antibody stain) in aortic root lesions. (B) Quantitative analysis of Mac-2-positive area in the aortic root lesions. (C) RT-PCR analysis of aortic expression of two macrophage markers (CD68 and Mac-2). RNA from the whole aortas was analyzed by qRT-PCR and normalized to GAPDH expression. (D) PPARδ agonist inhibits AngII-induced vascular inflammation. RNA levels in the aorta were analyzed by using qRT-PCR and normalized to GAPDH. Data are mean ± SD (n = 12 per group). *, P < 0.01 vs. HF/AngII by ANOVA.
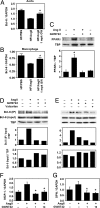
Fig. 3.
AngII suppresses Bcl-6 expression in the aorta and macrophage. RNA isolated from whole aorta (A) and peritoneal macrophages (B) was analyzed by qRT-PCR. Bcl-6 expression was normalized to GAPDH. Data are mean ± SD (n = 6–10 per group). *, P < 0.01 vs. HF/AngII by ANOVA. (C) AngII increases and PPARδ ligand decreases macrophage PPARδ protein expression. (D and E) Bcl-6 protein expression and PPARδ:Bcl-6 interaction in macrophages in response to AngII and/or GW0742. Bcl-6:PPARδ interaction was analyzed by Western blot analyses of total and PPARδ-bound Bcl-6 in macrophage nuclear proteins after pull-down assays. (F and G) MCP-1 and OPN mRNA levels in peritoneal macrophages treated with AngII and/or GW0742. Data are mean ± SD (n = 5 per group). *, P < 0.01 vs. HF/AngII by ANOVA.
Fig. 4.
PPARδ activation inhibits AngII-induced phosphorylation of MAP kinases. Mouse peritoneal macrophages stimulated with AngII in the presence or absence of GW0742 were analyzed by Western blot for MAP kinase activation. Activation of p38 (A) and ERK1/2 (B) was measured by the levels of phosphorylated p38 (pp38) and ERK1/2 (pERK1/2) normalized to total p38 and ERK1/2. n = 3 per group. *, P < 0.05 vs. AngII by ANOVA.
Fig. 5.
PPARδ activation inhibits AngII-mediated G protein signaling and suppression of RGS4 and RGS5 in macrophages. AngII infusion inhibits aorta RGS4 (A) and RGS5 (B) expression, and this effect is reversed by GW0742. Aorta RNA was analyzed by qRT-PCR, and RGS4 and RGS5 mRNA levels were normalized to GAPDH. Data are mean ± SD (n = 5 per group). *, P < 0.001 vs. HF/PBS; **, P < 0.05 vs. HF/AngII (by ANOVA).
Similar articles
- Peroxisome proliferator-activated receptor δ agonist GW1516 attenuates diet-induced aortic inflammation, insulin resistance, and atherosclerosis in low-density lipoprotein receptor knockout mice.
Bojic LA, Burke AC, Chhoker SS, Telford DE, Sutherland BG, Edwards JY, Sawyez CG, Tirona RG, Yin H, Pickering JG, Huff MW. Bojic LA, et al. Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):52-60. doi: 10.1161/ATVBAHA.113.301830. Epub 2013 Oct 24. Arterioscler Thromb Vasc Biol. 2014. PMID: 24158519 - Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice.
Matsushita Y, Ogawa D, Wada J, Yamamoto N, Shikata K, Sato C, Tachibana H, Toyota N, Makino H. Matsushita Y, et al. Diabetes. 2011 Mar;60(3):960-8. doi: 10.2337/db10-1361. Epub 2011 Jan 26. Diabetes. 2011. PMID: 21270242 Free PMC article. - The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE⁻/⁻ mice through inhibiting vascular inflammatory response.
Chen YX, Zhang M, Cai Y, Zhao Q, Dai W. Chen YX, et al. Biochem Biophys Res Commun. 2015 Oct 2;465(4):732-8. doi: 10.1016/j.bbrc.2015.08.066. Epub 2015 Aug 18. Biochem Biophys Res Commun. 2015. PMID: 26296466 - PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis.
Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, Zou Y, Hwang H, Kang H, Curtiss L, Evans RM, Lee CH. Barish GD, et al. Proc Natl Acad Sci U S A. 2008 Mar 18;105(11):4271-6. doi: 10.1073/pnas.0711875105. Epub 2008 Mar 12. Proc Natl Acad Sci U S A. 2008. PMID: 18337509 Free PMC article. - Peroxisome proliferator-activated receptor δ: a multifaceted metabolic player.
Bojic LA, Huff MW. Bojic LA, et al. Curr Opin Lipidol. 2013 Apr;24(2):171-7. doi: 10.1097/MOL.0b013e32835cc949. Curr Opin Lipidol. 2013. PMID: 23481229 Review.
Cited by
- The next generation of therapeutics for chronic kidney disease.
Breyer MD, Susztak K. Breyer MD, et al. Nat Rev Drug Discov. 2016 Aug;15(8):568-88. doi: 10.1038/nrd.2016.67. Epub 2016 May 27. Nat Rev Drug Discov. 2016. PMID: 27230798 Free PMC article. Review. - Loss of regulator of G protein signaling 5 exacerbates obesity, hepatic steatosis, inflammation and insulin resistance.
Deng W, Wang X, Xiao J, Chen K, Zhou H, Shen D, Li H, Tang Q. Deng W, et al. PLoS One. 2012;7(1):e30256. doi: 10.1371/journal.pone.0030256. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272317 Free PMC article. - PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation.
Varga T, Czimmerer Z, Nagy L. Varga T, et al. Biochim Biophys Acta. 2011 Aug;1812(8):1007-22. doi: 10.1016/j.bbadis.2011.02.014. Epub 2011 Mar 5. Biochim Biophys Acta. 2011. PMID: 21382489 Free PMC article. Review. - PPARs: Key Regulators of Airway Inflammation and Potential Therapeutic Targets in Asthma.
Banno A, Reddy AT, Lakshmi SP, Reddy RC. Banno A, et al. Nucl Receptor Res. 2018;5:101306. doi: 10.11131/2018/101306. Epub 2017 Dec 11. Nucl Receptor Res. 2018. PMID: 29450204 Free PMC article. - ERK and p38 Upregulation versus Bcl-6 Downregulation in Rat Kidney Epithelial Cells Exposed to Prolonged Hypoxia.
Luo F, Shi J, Shi Q, He X, Xia Y. Luo F, et al. Cell Transplant. 2017 Aug;26(8):1441-1451. doi: 10.1177/0963689717720296. Cell Transplant. 2017. PMID: 28901193 Free PMC article.
References
- Halkin A, Keren G. Potential indications for angiotensin-converting enzyme inhibitors in atherosclerotic vascular disease. Am J Med. 2002;112:126–134. - PubMed
- Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. - PubMed
- Dol F, et al. Angiotensin AT1 receptor antagonist irbesartan decreases lesion size, chemokine expression, and macrophage accumulation in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2001;38:395–405. - PubMed
- Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: The effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21:20–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- P01 HL088093/HL/NHLBI NIH HHS/United States
- R01 HL105278/HL/NHLBI NIH HHS/United States
- R37 DK057978/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous