Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice - PubMed (original) (raw)
doi: 10.1089/scd.2008.0018.
Huayan Hou, Terrence Town, Yuyan Zhu, Brian Giunta, Cyndy D Sanberg, Jin Zeng, Deyan Luo, Jared Ehrhart, Takashi Mori, Paul R Sanberg, Jun Tan
Affiliations
- PMID: 18366296
- PMCID: PMC2649688
- DOI: 10.1089/scd.2008.0018
Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice
William V Nikolic et al. Stem Cells Dev. 2008 Jun.
Abstract
Modulation of immune/inflammatory responses by diverse strategies including amyloid-beta (Abeta) immunization, nonsteroidal anti-inflammatory drugs, and manipulation of microglial activation states has been shown to reduce Alzheimer's disease (AD)-like pathology and cognitive deficits in AD transgenic mouse models. Human umbilical cord blood cells (HUCBCs) have unique immunomodulatory potential. We wished to test whether these cells might alter AD-like pathology after infusion into the PSAPP mouse model of AD. Here, we report a marked reduction in Abeta levels/beta-amyloid plaques and associated astrocytosis following multiple low-dose infusions of HUCBCs. HUCBC infusions also reduced cerebral vascular Abeta deposits in the Tg2576 AD mouse model. Interestingly, these effects were associated with suppression of the CD40-CD40L interaction, as evidenced by decreased circulating and brain soluble CD40L (sCD40L), elevated systemic immunoglobulin M (IgM) levels, attenuated CD40L-induced inflammatory responses, and reduced surface expression of CD40 on microglia. Importantly, deficiency in CD40 abolishes the effect of HUCBCs on elevated plasma Abeta levels. Moreover, microglia isolated from HUCBC-infused PSAPP mice demonstrated increased phagocytosis of Abeta. Furthermore, sera from HUCBC-infused PSAPP mice significantly increased microglial phagocytosis of the Abeta1-42 peptide while inhibiting interferon-gammainduced microglial CD40 expression. Increased microglial phagocytic activity in this scenario was inhibited by addition of recombinant CD40L protein. These data suggest that HUCBC infusion mitigates AD-like pathology by disrupting CD40L activity.
Figures
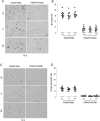
FIG. 1.
Cerebral A_β_/β_-amyloid pathology is reduced in PSAPP and Tg2576 mice peripherally infused with HUCBCs. Mouse paraffin-embedded coronal brain sections from the cingulate cortex (CC), hippocampus (H), and entorhinal cortex (EC) were stained with monoclonal human A_β antibody 4G8 (A) or Congo Red (C). Percentages (plaque area/total area) of A_β_ antibody-immunoreactive deposits (B) or of Congo Red-stained deposits (D) were calculated by quantitative image analysis (mean ± SD; n = 10, 5 females and 5 males per group). (E) A_β_ ELISA analysis was carried out for both levels of detergent-soluble A_β_1-40,42 (top panel) or 5 M guanidine-extracted A_β_1-40,42 (bottom panel). Data are represented as mean ± SD of A_β_1-40,42 (pg/mg protein). Mouse paraffin-embedded coronal brain sections from hippocampal regions of Tg2576 mice were stained with Congo Red (F). Positions of the hippocampal subfields CA1, CA3, and dentate gyrus (DG) are indicated (upper left panel). Arrows indicate A_β_ deposit-affected vessels. (G) Percentages (% labeled area) of Congo Red-stained plaques/vessels were quantified by image analysis (mean ± SD; n = 10, 5 females and 5 males), and percentage reduction is indicated.
FIG. 1.
Cerebral A_β_/β_-amyloid pathology is reduced in PSAPP and Tg2576 mice peripherally infused with HUCBCs. Mouse paraffin-embedded coronal brain sections from the cingulate cortex (CC), hippocampus (H), and entorhinal cortex (EC) were stained with monoclonal human A_β antibody 4G8 (A) or Congo Red (C). Percentages (plaque area/total area) of A_β_ antibody-immunoreactive deposits (B) or of Congo Red-stained deposits (D) were calculated by quantitative image analysis (mean ± SD; n = 10, 5 females and 5 males per group). (E) A_β_ ELISA analysis was carried out for both levels of detergent-soluble A_β_1-40,42 (top panel) or 5 M guanidine-extracted A_β_1-40,42 (bottom panel). Data are represented as mean ± SD of A_β_1-40,42 (pg/mg protein). Mouse paraffin-embedded coronal brain sections from hippocampal regions of Tg2576 mice were stained with Congo Red (F). Positions of the hippocampal subfields CA1, CA3, and dentate gyrus (DG) are indicated (upper left panel). Arrows indicate A_β_ deposit-affected vessels. (G) Percentages (% labeled area) of Congo Red-stained plaques/vessels were quantified by image analysis (mean ± SD; n = 10, 5 females and 5 males), and percentage reduction is indicated.
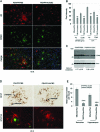
FIG. 2.
β_-Amyloid-associated microgliosis and astrocytosis are reduced in HUCBC infused-PSAPP mice. (A) Immunofluorescence was performed on mouse brain coronal paraffin sections prepared from PSAPP mice infused with HUCBC or PBS. The red signal indicates A_β+ (top panels); green indicates CD40+ (middle panels), and merged images (bottom panels) reveal co-localization of CD40 and A_β._ DAPI (blue) was used as a nuclear counterstain. (B) Immunofluorescence intensity for A_β_ and CD40 was determined. (C) Western blot analysis shows reduced CD40 expression in brain homogenates from PSAPP/HUCBC versus PSAPP/PBS mice as indicated (actin was used as an internal reference control). Densitometry analysis shows the ratio of CD40 to actin as indicated below the figure. (D) Immunohistochemistry analysis shows GFAP staining (top panel), and immunofluorescence (bottom panel) reveals co-localization of GFAP (red signal) and A_β_ (green signal). (E) Morphometric analysis results (mean GFAP/β_-amyloid double positive plaques per mouse ± SD) are shown for the neocortex and the hippocampus of PSAPP/HUCBC versus PSAPP/PBS mice. Percent reduction of plaques double positive for GFAP and A_β in PSAPP/HUCBC mice is indicated.
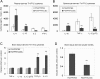
FIG. 3.
HUCBC infusion results in CD40-dependent increased plasma A_β_ levels in PSAPP mice. ELISA analysis results are shown from blood (plasma) for A_β_1-40 (A), A_β_1-42 (B), sCD40L (C), and IgM/IgG (D). Data are presented as mean ± SD (n = 10) for A_β_1-40, A_β_1-42, or sCD40L (pg/ml plasma). Arrows below the panels show the time for each peripheral infusion with HUCBC or PBS. (D) Data are presented as a ratio of IgM to IgG in blood (plasma) from mice at the 6th month following the treatment. A_β_ ELISA analysis for A_β_1-40 (E) and A_β_1-42 (F) in blood (plasma) derived from PSAPP/CD40+/+ or PSAPP/CD40−/− mice at the 2nd month, following the third HUCBC infusion. Data in E and F are presented as mean ± SD (n = 4, 2 males and 2 females) of A_β_1-40 or A_β_1-42 (pg/ml plasma).
FIG. 4.
HUCBC infusion promotes anti-inflammatory/Th2 responses and decreases sCD40L in the CNS. ELISA results are shown for plasma-derived (A), splenocyte culture-derived (B), brain tissue-derived cytokines (C), and brain tissue-derived sCD40L (D). Data are presented as mean ± SD (n = 10) values of cytokines (pg/ml plasma or medium) (A and B) or fold increase of cytokines over control (untreated) mice (C and D).
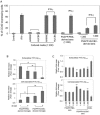
FIG. 5.
HUCBCs modulate microglial CD40 expression and promote A_β_ microglial/macrophage phagocytic activity. (A) FACS analysis for CD40 expression in primary wild-type neonatal microglial cells treated with cultured medium from HUCBCs or human adult mononuclear cells (HAMNCs), or serum from individual PSAPP/HUCBC or PSAPP/PBS mice following IFN-γ challenge. Data are presented as percentage of CD40 expressing cells (mean ± SD; n = 5). (B–D) Microglial/macrophage phagocytosis assay results for extracellular and cell-associated FITC-A_β_1-42, which was detected using a fluorometer. Data are represented as the relative fold of mean ± SD fluorescence over control for each sample (n = 4 for each condition presented). Primary microglial cells from adult PSAPP/HUCBC or PSAPP/PBS mice (B), wild-type neonatal microglia (C), and primary peripheral macrophages (D) are shown.
FIG. 5.
HUCBCs modulate microglial CD40 expression and promote A_β_ microglial/macrophage phagocytic activity. (A) FACS analysis for CD40 expression in primary wild-type neonatal microglial cells treated with cultured medium from HUCBCs or human adult mononuclear cells (HAMNCs), or serum from individual PSAPP/HUCBC or PSAPP/PBS mice following IFN-γ challenge. Data are presented as percentage of CD40 expressing cells (mean ± SD; n = 5). (B–D) Microglial/macrophage phagocytosis assay results for extracellular and cell-associated FITC-A_β_1-42, which was detected using a fluorometer. Data are represented as the relative fold of mean ± SD fluorescence over control for each sample (n = 4 for each condition presented). Primary microglial cells from adult PSAPP/HUCBC or PSAPP/PBS mice (B), wild-type neonatal microglia (C), and primary peripheral macrophages (D) are shown.
Similar articles
- CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation.
Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J. Obregon D, et al. Neurobiol Dis. 2008 Feb;29(2):336-53. doi: 10.1016/j.nbd.2007.09.009. Epub 2007 Oct 16. Neurobiol Dis. 2008. PMID: 18055209 Free PMC article. - Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice.
Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Tan J, et al. Nat Neurosci. 2002 Dec;5(12):1288-93. doi: 10.1038/nn968. Nat Neurosci. 2002. PMID: 12402041 - Identification of CD40 ligand in Alzheimer's disease and in animal models of Alzheimer's disease and brain injury.
Calingasan NY, Erdely HA, Altar CA. Calingasan NY, et al. Neurobiol Aging. 2002 Jan-Feb;23(1):31-9. doi: 10.1016/s0197-4580(01)00246-9. Neurobiol Aging. 2002. PMID: 11755016 - Impact of the CD40-CD40L dyad in Alzheimer's disease.
Giunta B, Rezai-Zadeh K, Tan J. Giunta B, et al. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):149-55. doi: 10.2174/187152710791012099. CNS Neurol Disord Drug Targets. 2010. PMID: 20205645 Free PMC article. Review. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
- Human umbilical cord blood-derived monocytes improve cognitive deficits and reduce amyloid-β pathology in PSAPP mice.
Darlington D, Li S, Hou H, Habib A, Tian J, Gao Y, Ehrhart J, Sanberg PR, Sawmiller D, Giunta B, Mori T, Tan J. Darlington D, et al. Cell Transplant. 2015;24(11):2237-50. doi: 10.3727/096368915X688894. Epub 2015 Jul 30. Cell Transplant. 2015. PMID: 26230612 Free PMC article. - Multiple low-dose infusions of human umbilical cord blood cells improve cognitive impairments and reduce amyloid-β-associated neuropathology in Alzheimer mice.
Darlington D, Deng J, Giunta B, Hou H, Sanberg CD, Kuzmin-Nichols N, Zhou HD, Mori T, Ehrhart J, Sanberg PR, Tan J. Darlington D, et al. Stem Cells Dev. 2013 Feb 1;22(3):412-21. doi: 10.1089/scd.2012.0345. Epub 2012 Sep 5. Stem Cells Dev. 2013. PMID: 22816379 Free PMC article. - Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques.
Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW, Lee JI, Na DL, Yang YS, Oh W, Chang JW. Kim JY, et al. Cell Death Differ. 2012 Apr;19(4):680-91. doi: 10.1038/cdd.2011.140. Epub 2011 Oct 21. Cell Death Differ. 2012. PMID: 22015609 Free PMC article. - LISPRO mitigates β-amyloid and associated pathologies in Alzheimer's mice.
Habib A, Sawmiller D, Li S, Xiang Y, Rongo D, Tian J, Hou H, Zeng J, Smith A, Fan S, Giunta B, Mori T, Currier G, Shytle DR, Tan J. Habib A, et al. Cell Death Dis. 2017 Jun 15;8(6):e2880. doi: 10.1038/cddis.2017.279. Cell Death Dis. 2017. PMID: 28617434 Free PMC article. Retracted. - The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders.
Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. Borlongan CV, et al. Prog Neurobiol. 2011 Oct;95(2):213-28. doi: 10.1016/j.pneurobio.2011.08.005. Epub 2011 Aug 30. Prog Neurobiol. 2011. PMID: 21903148 Free PMC article. Review.
References
- Benzing WC. Wujek JR. Ward EK. Shaffer D. Ashe KH. Younkin SG. Brunden KR. Evidence for glialmediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. - PubMed
- Bard F. Cannon C. Barbour R. Burke RL. Games D. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Lieberburg I. Motter R. Nguyen M. Soriano F. Vasquez N. Weiss K. Welch B. Seubert P. Schenk D. Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 2000;6:916–919. - PubMed
- Nicoll JA. Wilkinson D. Holmes C. Steart P. Markham H. Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nature Med. 2003;9:448–452. - PubMed
- Schenk D. Barbour R. Dunn W. Gordon G. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Liao Z. Lieberburg I. Motter R. Mutter L. Soriano F. Shopp G. Vasquez N. Vandevert C. Walker S. Wogulis M. Yednock T. Games D. Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 AG029726/AG/NIA NIH HHS/United States
- R01 AG032432/AG/NIA NIH HHS/United States
- R41AG031586/AG/NIA NIH HHS/United States
- R01NS048335/NS/NINDS NIH HHS/United States
- K99 AG029726/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials