Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response - PubMed (original) (raw)
Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response
Alexander Swarbrick et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Recent evidence demonstrates that senescence acts as a barrier to tumorigenesis in response to oncogene activation. Using a mouse model of breast cancer, we tested the importance of the senescence response in solid cancer and identified genetic pathways regulating this response. Mammary expression of activated Ras led to the formation of senescent cellular foci in a majority of mice. Deletion of the p19(ARF), p53, or p21(WAF1) tumor suppressors but not p16(INK4a) prevented senescence and permitted tumorigenesis. Id1 has been implicated in the control of senescence in vitro, and elevated expression of Id1 is found in a number of solid cancers, so we tested whether overexpression of Id1 regulates senescence in vivo. Although overexpression of Id1 in the mammary epithelium was not sufficient for tumorigenesis, mice with expression of both Id1 and activated Ras developed metastatic cancer. These tumors expressed high levels of p19(Arf), p53, and p21(Waf1), demonstrating that Id1 acts to make cells refractory to p21(Waf1)-dependent cell cycle arrest. Inactivation of the conditional Id1 allele in established tumors led to widespread senescence within 10 days, tumor growth arrest, and tumor regression in 40% of mice. Mice in which Id1 expression was inactivated also exhibited greatly reduced pulmonary metastatic load. These data demonstrate that established tumors remain sensitive to senescence and that Id1 may be a valuable target for therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
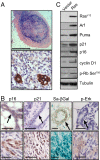
Ras activation triggers cellular senescence in vivo via p19ARF-p53-p21waf1 signaling. (A and B) Four weeks after transplantation, mammary fat pads of mice transplanted with MMECs expressing oncogenic Ras were harvested and sectioned. (A) Foci at the site of injection were stained with H&E (Upper) or an antibody to cytokeratins (Lower). (Scale bars: Upper, 1 mm; Lower, 100 μm.) (B) Ras-expressing glands (Lower) or controls (Upper) were stained for SA-βGal activity and by IHC for _p_-Erk1/2, p21waf1, and p16INK4A. Arrows indicate mammary ducts. (Scale bars: 100 μm.) (C) Seven days after infection and antibiotic selection in vitro, MMECs expressing oncogenic Ras or vector control were harvested, and extracts were analyzed by Western blot for components of the p16INK4A and p19ARF–p53 pathways.
Fig. 2.
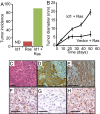
p19ARF, p53, and p21waf1 but not p16INK4A are required to suppress Ras-dependent tumorigenesis and senescence. (A) MMECs nullizygous for the indicated gene, or wild-type controls, were transduced with oncogenic Ras and transplanted to the cleared fat pads of naive hosts. Graph shows tumor incidence (>2 mm) 5 weeks after transplantation. n > 9 for each group. (B) _p16ink4a_−/− MMECs were transduced with oncogenic Ras and 4 weeks after mammary fat pad transplantation and stained for SA-βGal activity. S, stroma; T, transplanted cells. (Scale bar: 100 μm.) (C) _p19arf_−/− MMECs were transduced with oncogenic Ras in vitro, and extracts were analyzed by Western blot for RasV12, p16INK4A, p21waf1, and tubulin.
Fig. 3.
Id1 and oncogenic Ras cooperate in mammary tumorigenesis. Mice received transplants of mammary epithelial cells (MMECs) transduced with oncogenic Ras, Id1 or oncogenic Ras + Id1. (A) Incidence of mammary tumors 6 weeks after transplant. n > 10 for all groups. ND, not detected (B) Diameter of tumors over time after transplantation. Error bars represent mean diameters ± SEM. n > 10 for both groups. (C–E) Sections from a tumor expressing oncogenic Ras and Id1 stained with hematoxylin and eosin C or antibodies to cytokeratins 14 (D) or 18 (E). (F–H) IHC for p21waf1 on sections from a focus of epithelium expressing activated Ras (F), a tumor derived from activated Ras expression in tp53 null MMECs (G), or a tumor expressing activated Ras and Id1 (H). (Scale bars: 100 μm.)
Fig. 4.
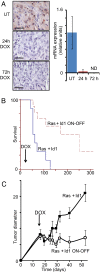
Sustained Ras activity is required for tumor survival. Mice received transplants of MMECs transduced with Id1 and the tetracycline transactivator (tTA), both expressed constitutively under the control of the MSCV LTR, in addition to oncogenic Ras under the control of the TREti conditional promoter. Twenty-one days after transplantation, mice received doxycycline (DOX) by a single IP injection and in their food to inactivate the hrasV12 transgene. (A) Immunohistochemical staining for _p_-Erk of tumor extracts harvested 0, 24, or 72 h after doxycycline administration. (B) mRNA expression of the hrasV12 transgene, Egr1, and cyclin D1 were determined by quantitative PCR, using RNA isolated from tumors 0, 24, or 72 h after doxycycline administration. Errors bars represent mean values ± SEM. (C) Tumor diameter in mice receiving doxycycline at day 21 (Id1+ Ras ON-OFF) or controls (Id1 + Ras) was measured over time. Error bars represent mean values ± SEM. (D and E) Sections of tumors from mice treated with doxycycline for 3 days (E) or controls (D) were stained with antibodies to activated caspase 3 (brown foci) or cleaved cytokeratin (M30 cytodeath). (Scale bars: 100 μm.)
Fig. 5.
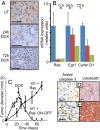
Sustained Id1 expression is required for tumor growth. Mice received transplants of MMECs transduced with activated Ras and the tetracycline transactivator (tTA), both expressed constitutively under the control of the MSCV LTR, in addition to Id1 under the control of the TREti conditional promoter. Nineteen days after transplantation, mice received doxycycline by a single IP injection and in their food. (A) Analysis of tumor extracts harvested 0, 24, or 72 h after doxycycline administration by IHC, using Id1 antisera and by RT- PCR for the Id1 transgene. Errors bars represent mean values ± SEM. (B) Inactivation of the Id1 transgene at day 19 extends median time to ethical end point of mice from 66 days to 253 days. Kaplan–Meyer analysis, P < 0.003 (C) Tumor diameters in mice receiving doxycycline in their food (Ras + Id1 ON-OFF) or controls (Ras + Id1) were measured over time. Errors bars represent mean values ± SEM. Forty percent of tumors regressed to an undetectable state by day 80. n = 5–8 mice per time point for both groups. Statistical comparison (t test) between groups at each time point: P < 0.01 at days 29, 32, 40, and 53. (Scale bars: 100 μm.)
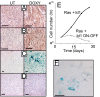
Fig. 6.
Id1 regulates senescence in vivo. (A–D) Sections of tumors from mice treated with doxycycline or controls were stained for caspase-cleaved cytokeratin (D) (3 days Doxy), SA-βGal (C), p16INK4A (B), and DcR2 (A). (A–C) 10 days Doxy. (E and F) Cells from control tumors were cultured and treated with doxycycline at day 5 to inactivate the id1 transgene. (E) Cell number was measured over time after doxycycline administration. Cells were passaged every 6–8 days. (F) SA-βGal staining of cells 7 days after doxycycline administration. (Scale bars: 100 μm.)
Similar articles
- Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis.
Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Sarkisian CJ, et al. Nat Cell Biol. 2007 May;9(5):493-505. doi: 10.1038/ncb1567. Epub 2007 Apr 22. Nat Cell Biol. 2007. PMID: 17450133 - Resistance of primary cultured mouse hepatic tumor cells to cellular senescence despite expression of p16(Ink4a), p19(Arf), p53, and p21(Waf1/Cip1).
Obata M, Imamura E, Yoshida Y, Goto J, Kishibe K, Yasuda A, Ogawa K. Obata M, et al. Mol Carcinog. 2001 Sep;32(1):9-18. doi: 10.1002/mc.1059. Mol Carcinog. 2001. PMID: 11568971 - Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.
Shin J, Yang J, Lee JC, Baek KH. Shin J, et al. Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963 - Tumor suppressors and oncogenes in cellular senescence.
Bringold F, Serrano M. Bringold F, et al. Exp Gerontol. 2000 May;35(3):317-29. doi: 10.1016/s0531-5565(00)00083-8. Exp Gerontol. 2000. PMID: 10832053 Review. - Replicative senescence as a barrier to human cancer.
Parkinson EK, Munro J, Steeghs K, Morrison V, Ireland H, Forsyth N, Fitzsimmons S, Bryce S. Parkinson EK, et al. Biochem Soc Trans. 2000 Feb;28(2):226-33. doi: 10.1042/bst0280226. Biochem Soc Trans. 2000. PMID: 10816133 Review.
Cited by
- Inhibition of BMP and of TGFβ receptors downregulates expression of XIAP and TAK1 leading to lung cancer cell death.
Augeri DJ, Langenfeld E, Castle M, Gilleran JA, Langenfeld J. Augeri DJ, et al. Mol Cancer. 2016 Apr 6;15:27. doi: 10.1186/s12943-016-0511-9. Mol Cancer. 2016. PMID: 27048361 Free PMC article. - Oncogene-induced senescence and its role in tumor suppression.
Reddy JP, Li Y. Reddy JP, et al. J Mammary Gland Biol Neoplasia. 2011 Sep;16(3):247-56. doi: 10.1007/s10911-011-9221-5. Epub 2011 Jun 18. J Mammary Gland Biol Neoplasia. 2011. PMID: 21681694 Review. - The role of p21 in regulating mammalian regeneration.
Arthur LM, Heber-Katz E. Arthur LM, et al. Stem Cell Res Ther. 2011 Jun 29;2(3):30. doi: 10.1186/scrt71. Stem Cell Res Ther. 2011. PMID: 21722344 Free PMC article. - Ras oncogene-independent activation of RALB signaling is a targetable mechanism of escape from NRAS(V12) oncogene addiction in acute myeloid leukemia.
Pomeroy EJ, Lee LA, Lee RDW, Schirm DK, Temiz NA, Ma J, Gruber TA, Diaz-Flores E, Moriarity BS, Downing JR, Shannon KM, Largaespada DA, Eckfeldt CE. Pomeroy EJ, et al. Oncogene. 2017 Jun 8;36(23):3263-3273. doi: 10.1038/onc.2016.471. Epub 2016 Dec 19. Oncogene. 2017. PMID: 27991934 Free PMC article. - Interleukin-27 signaling promotes immunity against endogenously arising murine tumors.
Natividad KD, Junankar SR, Mohd Redzwan N, Nair R, Wirasinha RC, King C, Brink R, Swarbrick A, Batten M. Natividad KD, et al. PLoS One. 2013;8(3):e57469. doi: 10.1371/journal.pone.0057469. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23554861 Free PMC article.
References
- Campisi J, d'Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. - PubMed
- Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. - PubMed
- Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. - PubMed
- Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous