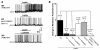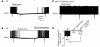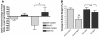Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice - PubMed (original) (raw)
Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice
Jennifer W Hill et al. J Clin Invest. 2008 May.
Abstract
Normal food intake and body weight homeostasis require the direct action of leptin on hypothalamic proopiomelanocortin (POMC) neurons. It has been proposed that leptin action requires PI3K activity. We therefore assessed the contribution of PI3K signaling to leptin's effects on POMC neurons and organismal energy balance. Leptin caused a rapid depolarization of POMC neurons and an increase in action potential frequency in patch-clamp recordings of hypothalamic slices. Pharmacologic inhibition of PI3K prevented this depolarization and increased POMC firing rate, indicating a PI3K-dependent mechanism of leptin action. Mice with genetically disrupted PI3K signaling in POMC cells failed to undergo POMC depolarization or increased firing frequency in response to leptin. Insulin's ability to hyperpolarize POMC neurons was also abolished in these mice. Moreover, targeted disruption of PI3K blunted the suppression of feeding elicited by central leptin administration. Despite these differences, mice with impaired PI3K signaling in POMC neurons exhibited normal long-term body weight regulation. Collectively, these results suggest that PI3K signaling in POMC neurons is essential for leptin-induced activation and insulin-induced inhibition of POMC cells and for the acute suppression of food intake elicited by leptin, but is not a major contributor to the regulation of long-term organismal energy homeostasis.
Figures
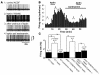
Figure 1. Leptin increases POMC neuronal activity in a PI3K-dependent manner.
(A) Sample traces of loose-patch recording in control ACSF, with leptin (100 nM), after wash to control ACSF, and in the presence of the PI3K inhibitor wortmannin (100 nM). (B) Histogram showing spike frequency of the same neuron in A organized into 20-s epochs. (C) Leptin-induced increase in neuronal activity of identified POMC neurons. P values compared with control ACSF are shown.
Figure 2. Leptin causes depolarization of POMC neurons via a PI3K-dependent mechanism.
(A) Current-clamp recordings demonstrated that disruption of PI3K signaling occluded the leptin-induced depolarization of POMC neurons from rest. Top: Characteristic leptin-induced (100 nM) depolarization of WT neurons. Middle: Absence of leptin-induced depolarization in POMC neurons from Pik3r1 POMCKO Pik3r2–/– mice. Bottom: Leptin failed to depolarize POMC neurons in the presence of the PI3K inhibitor LY294002 (10 μM). (B) Leptin-induced responses of identified POMC neurons from WT, Pik3r1 POMCKO, and Pik3r1 POMCKO Pik3r2–/– mice. P values compared with WT are shown.
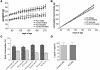
Figure 3. Leptin induces a decrease in whole-cell input resistance in POMC neurons.
(A) Current-clamp recording from a WT neuron showing decreased voltage deflection and increased action potential frequency after leptin application (100 nM). (B) Current versus voltage (I-V) plot from same WT neuron illustrating a characteristic decrease in input resistance subsequent to leptin application. Shown are responses before (control) and during leptin application. (C) Decreased whole-cell input resistance from the neuron groups examined. P values compared with WT treated with leptin alone are shown.
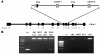
Figure 4. Deletion of exon 7 of Pik3r1 in genomic DNA from hypothalamic nuclei of Pik3r1 POMCKO Pik3r2–/– mice.
(A) The 1,275-bp region between primer binding sites becomes 298 bp in length when exon 7 is excised by cre recombinase. (B) Amplified genomic DNA from a Pik3r1 POMCKO Pik3r2–/– and littermate control (Con) mice. Tail DNA from a Pik3r1–/– mouse is shown as a positive control (+). Olf, olfactory bulb DNA; HYP, DNA from whole hypothalamus; DMH, dorsomedial hypothalamic nucleus; PVN, paraventricular nucleus.
Figure 5. Insulin hyperpolarizes POMC neurons via a PI3K-dependent mechanism.
(A) Current-clamp record depicting the hyperpolarization of a POMC neuron from rest by 50 nM insulin. The hyperpolarization was reversible within 15 min of wash to control ACSF. Downward deflections are responses to rectangular current steps. (B) Current-clamp recording at resting membrane potential showing insulin-induced hyperpolarization (50 nM) followed by tolbutamide (200 μM) blockade of the insulin effect. (C) Sample trace illustrating the absence of insulin-induced hyperpolarization in POMC neurons from Pik3r1 POMCKO Pik3r2–/– mice. (D) Insulin-induced responses of identified POMC neurons from WT and Pik3r1 POMCKO Pik3r2–/– mice. P = 0.02, Pik3r1 POMCKO Pik3r2–/– versus WT.
Figure 6. Leptin fails to suppress feeding in Pik3r1 POMCKO Pik3r2–/– mice.
(A) Food consumption was measured 3 and 24 hours after leptin administration and compared with food consumption in each animal following saline administration. Values were normalized to kg body weight (n = 11–15). (B) Body weight 24 hours after injection in Pik3r1 POMCKO Pik3r2–/– mice and littermate controls lacking POMC-cre expression. Data are mean ± SEM. *P = 0.0487, **P = 0.0022 versus saline-injected controls; Student’s t test.
Figure 7. Energy homeostasis, fertility, and linear growth.
(A) Body weight curves and (B) cumulative food intake of Pik3r1 POMCKO Pik3r2–/– mice and littermate controls lacking POMC-cre expression on high-fat chow. (C) Body fat percentage and lean mass in 8-month-old male and female diet-induced obese mice. (D) Serum leptin levels in 12-week-old male mice fed normal chow.
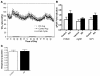
Figure 8. Metabolic rate and hypothalamic gene expression in mice lacking p85 in POMC neurons.
(A) Oxygen consumption and (C) respiratory quotient was measured by indirect calorimetry and averaged over a 48-hour time period in male 8-week-old Pik3r1 POMCKO Pik3r2–/– mice and littermate controls lacking POMC-cre expression (n = 8). No significant difference was observed between groups. (B) NPY, AgRP, and POMC expression in hypothalami from 10-month-old male control (n = 7) or Pik3r1 POMCKO Pik3r2–/– mice (n = 5) as measured by quantitative PCR.
Figure 9. Model of leptin action in the arcuate POMC neuron.
Acute stimulation of the long-form leptin receptor results in activation of the receptor-associated tyrosine kinase JAK2, which phosphorylates IRS proteins and in turn activates PI3K. PI3K subsequently activates a putative mixed-cation channel, which leads to depolarization and increased firing frequency responsible for POMC-mediated acute leptin actions. Sustained stimulation of the leptin receptor recruits SH2-containing tyrosine phosphatase (SHP2) to the leptin receptor tyrosine 985, which in turn regulates ERK activation. In addition, tyrosine 1138 of the leptin receptor recruits STAT3 and regulates transcriptional events such as the induction of SOCS3 required for long-term regulation of appetite and energy expenditure by leptin. The model predicts that PI3K is necessary for the acute actions of leptin in arcuate POMC neurons. However, other signaling cascades such as MAPK,SHP2, and STAT are likely candidates for the leptin-induced changes in long-term energy balance (9, 51, 63).
Similar articles
- Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity.
Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Münzberg H, Hampel B, Alber J, Kloppenburg P, Brüning JC, Wunderlich FT. Ernst MB, et al. J Neurosci. 2009 Sep 16;29(37):11582-93. doi: 10.1523/JNEUROSCI.5712-08.2009. J Neurosci. 2009. PMID: 19759305 Free PMC article. - Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis.
Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Hill JW, et al. Endocrinology. 2009 Nov;150(11):4874-82. doi: 10.1210/en.2009-0454. Epub 2009 Oct 9. Endocrinology. 2009. PMID: 19819947 Free PMC article. - PI3K integrates the action of insulin and leptin on hypothalamic neurons.
Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. Xu AW, et al. J Clin Invest. 2005 Apr;115(4):951-8. doi: 10.1172/JCI24301. Epub 2005 Mar 10. J Clin Invest. 2005. PMID: 15761497 Free PMC article. - Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis.
Mela V, Vargas A, Meza C, Kachani M, Wagner EJ. Mela V, et al. J Steroid Biochem Mol Biol. 2016 Jun;160:15-26. doi: 10.1016/j.jsbmb.2015.07.014. Epub 2015 Jul 29. J Steroid Biochem Mol Biol. 2016. PMID: 26232394 Free PMC article. Review. - Do POMC neurons have a sweet tooth for leptin? Special issue: Role of nutrients in nervous control of energy balance.
Srour N, Caron A, Michael NJ. Srour N, et al. Biochimie. 2024 Aug;223:179-187. doi: 10.1016/j.biochi.2022.09.006. Epub 2022 Sep 17. Biochimie. 2024. PMID: 36122808 Review.
Cited by
- Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons.
Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ, Li D, Burrin DG, Chan L, Guan X. Shi X, et al. Cell Metab. 2013 Jul 2;18(1):86-98. doi: 10.1016/j.cmet.2013.06.014. Cell Metab. 2013. PMID: 23823479 Free PMC article. - The Role of Cdc42 in the Insulin and Leptin Pathways Contributing to the Development of Age-Related Obesity.
Umbayev B, Saliev T, Safarova Yantsen Y, Yermekova A, Olzhayev F, Bulanin D, Tsoy A, Askarova S. Umbayev B, et al. Nutrients. 2023 Nov 29;15(23):4964. doi: 10.3390/nu15234964. Nutrients. 2023. PMID: 38068822 Free PMC article. Review. - Estradiol signaling in the regulation of reproduction and energy balance.
Sinchak K, Wagner EJ. Sinchak K, et al. Front Neuroendocrinol. 2012 Oct;33(4):342-63. doi: 10.1016/j.yfrne.2012.08.004. Epub 2012 Sep 7. Front Neuroendocrinol. 2012. PMID: 22981653 Free PMC article. Review. - Unravelling the mysterious roles of melanocortin-3 receptors in metabolic homeostasis and obesity using mouse genetics.
Girardet C, Begriche K, Ptitsyn A, Koza RA, Butler AA. Girardet C, et al. Int J Obes Suppl. 2014 Jul;4(Suppl 1):S37-44. doi: 10.1038/ijosup.2014.10. Epub 2014 Jul 8. Int J Obes Suppl. 2014. PMID: 27152165 Free PMC article. Review. - Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons.
Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK. Sohn JW, et al. Mol Metab. 2016 Jun 14;5(8):669-679. doi: 10.1016/j.molmet.2016.06.004. eCollection 2016 Aug. Mol Metab. 2016. PMID: 27656404 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK53301-07S2/DK/NIDDK NIH HHS/United States
- F32 DK077487/DK/NIDDK NIH HHS/United States
- R37 DK053301/DK/NIDDK NIH HHS/United States
- R37 GM041890/GM/NIGMS NIH HHS/United States
- GM41890/GM/NIGMS NIH HHS/United States
- 1F32DK066972/DK/NIDDK NIH HHS/United States
- R01 GM041890/GM/NIGMS NIH HHS/United States
- R01 DK053301/DK/NIDDK NIH HHS/United States
- 1F32DK077487/DK/NIDDK NIH HHS/United States
- P01 DK56116/DK/NIDDK NIH HHS/United States
- F32 DK066972/DK/NIDDK NIH HHS/United States
- P01 DK056116/DK/NIDDK NIH HHS/United States
- DK53301/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous