Brain chromatin remodeling: a novel mechanism of alcoholism - PubMed (original) (raw)
Brain chromatin remodeling: a novel mechanism of alcoholism
Subhash C Pandey et al. J Neurosci. 2008.
Abstract
The treatment of alcoholism requires the proper management of ethanol withdrawal symptoms, such as anxiety, to prevent further alcohol use and abuse. In this study, we investigated the potential role of brain chromatin remodeling, caused by histone modifications, in alcoholism. We found that the anxiolytic effects produced by acute alcohol were associated with a decrease in histone deacetylase (HDAC) activity and increases in acetylation of histones (H3 and H4), levels of CREB (cAMP-responsive element binding) binding protein (CBP), and neuropeptide Y (NPY) expression in the amygdaloid brain regions of rats. However, the anxiety-like behaviors during withdrawal after chronic alcohol exposure were associated with an increase in HDAC activity and decreases in acetylation of H3 and H4, and levels of both CBP and NPY in the amygdala. Blocking the observed increase in HDAC activity during alcohol withdrawal with the HDAC inhibitor, trichostatin A, rescued the deficits in H3 and H4 acetylation and NPY expression (mRNA and protein levels) in the amygdala (central and medial nucleus of amygdala) and prevented the development of alcohol withdrawal-related anxiety in rats as measured by the elevated plus maze and light/dark box exploration tests. These results reveal a novel role for amygdaloid chromatin remodeling in the process of alcohol addiction and further suggest that HDAC inhibitors may be potential therapeutic agents in treating alcohol withdrawal symptoms.
Figures
Figure 1.
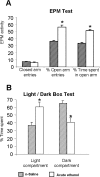
A, B, The effect of acute ethanol exposure (1 h after 1 g/kg; i.p.) on open-arm and closed-arm activity in the EPM test (A) and on LDB exploration test (B) for anxiety-like behaviors. Values are the mean ± SEM of 11–12 rats in each group. *Significantly different from n-saline-treated rats (p < 0.01–0.001).
Figure 2.
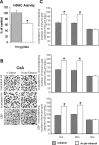
A, Acute ethanol exposure inhibited HDAC activity in the amygdala of rats [injected with ethanol (1 h after 1 g/kg, i.p.) or n-saline]. The HDAC activity was determined in the cell lysates of amygdala by measuring the deacetylation of acetylated lysine side chains. The values are the mean ± SEM of seven rats per group. *Significantly different from n-saline-treated rats (p < 0.01; Student's t test). B, Low-magnification photomicrographs of acetylated histones H3 (Lys 9) and H4 (Lys 8) and CBP gold-immunolabeling (protein levels) in central amygdaloid (CeA) structures of n-saline or acute-ethanol-treated rats. Scale bar, 40 μm. C, Effect of acute ethanol treatment on protein levels of acetylated H3 and H4, and of CBP in various amygdaloid (CeA, MeA, and BLA) structures of rats. Values are the mean ± SEM of seven to nine rats per group. *Significantly different from the n-saline-treated rats (p < 0.001; Student's t test).
Figure 3.
A, Low-magnification photomicrographs of NPY mRNA (in situ RT-PCR) and NPY gold-immunolabeling (protein) in CeA of n-saline and acute ethanol-treated rats. Scale bar, 40 μm. B, Effect of acute ethanol treatment on mRNA and protein levels of NPY in CeA, MeA, and BLA of rats. Values are the mean ± SEM of five rats per group. *Significantly different from the n-saline-treated rats (p < 0.001; Student's t test).
Figure 4.
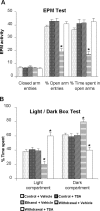
A, Effect of TSA treatment on open-arm and closed-arm activities in the EPM test for anxiety-like behaviors during ethanol withdrawal. The control diet-fed, ethanol diet-fed (0 h withdrawal), and ethanol diet-fed (24 h withdrawal) rats were injected with TSA (2 mg/kg, i.p.) or vehicle once, 2 h before measuring anxiety-like behaviors. The groups are represented as follows: control diet-fed + vehicle = control + vehicle; control diet-fed + TSA = control + TSA; ethanol diet-fed (0 h withdrawal) + vehicle = ethanol + vehicle; ethanol diet-fed (24 h withdrawal) + vehicle = withdrawal + vehicle; ethanol diet-fed (24 h withdrawal) + TSA = withdrawal + TSA. Values are the mean ± SEM of eight to nine rats per group. *Significantly different from control diet-fed rats treated with vehicle (p < 0.001; ANOVA followed by Tukey's test). B, Effect of TSA treatment on LDB exploration test for anxiety-like behaviors during ethanol withdrawal after chronic ethanol exposure. Values are the mean ± SEM of nine rats per group. *Significantly different from control diet-fed rats treated with vehicle (p < 0.01–0.001; ANOVA followed by Tukey's test).
Figure 5.
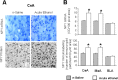
A, Effect of ethanol withdrawal after chronic ethanol treatment and effect of TSA treatment on HDAC activity in the amygdala of rats. The HDAC activity was determined in the cell lysates of amygdala by measuring the deacetylation of acetylated lysine side chains. Values are the mean ± SEM of seven rats per group. *Significantly different from control diet-fed rats treated with vehicle (p < 0.05; ANOVA followed by Tukey's test). B, Low-magnification photomicrographs of acetylated histones H3 (Lys 9) and H4 (Lys 8), and CBP gold-immunolabeling (protein levels) in the CeA of control diet-fed, ethanol-fed, and ethanol-withdrawn rats treated with TSA or vehicle. Scale bar, 40 μm. The groups are represented as follows: control diet-fed + vehicle = control + vehicle; control diet-fed + TSA = control + TSA; ethanol diet-fed (0 h withdrawal) + vehicle = ethanol + vehicle; ethanol diet-fed (24 h withdrawal) + vehicle = withdrawal + vehicle; ethanol diet-fed (24 h withdrawal) + TSA = withdrawal + TSA. C, Effect of TSA treatment on protein levels of acetylated H3 and H4, and CBP in the CeA, MeA, and BLA of rats during ethanol withdrawal after chronic ethanol exposure. Values are the mean ± SEM of five to six rats per group. *Significantly different from the control diet-fed rats treated with vehicle (p < 0.001; ANOVA followed by Tukey's test).
Figure 6.
A, Low-magnification photomicrographs of NPY mRNA and NPY gold-immunolabeling (protein) in the CeA of control diet-fed, ethanol diet-fed, and ethanol-withdrawn rats treated with TSA or vehicle. Scale bar, 40 μm. The groups are represented as follows: control diet-fed + vehicle = control + vehicle; control diet-fed + TSA = control + TSA; ethanol diet-fed (0 h withdrawal) + vehicle = ethanol + vehicle; ethanol diet-fed (24 h withdrawal) + vehicle = withdrawal + vehicle; ethanol diet-fed (24 h withdrawal) + TSA = withdrawal + TSA. B, Effect of TSA treatment on protein and mRNA levels of NPY in the CeA, MeA, and BLA of rats during ethanol withdrawal after chronic ethanol exposure. Values are the mean ± SEM of five to six rats per group. *Significantly different from the control diet-fed rats treated with vehicle (p < 0.001; ANOVA followed by Tukey's test).
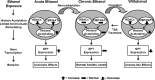
Figure 7.
Proposed model for chromatin remodeling in the central and medial nucleus of amygdala during acute and chronic ethanol exposure. The present investigation reveals that increased histone acetylation caused by either inhibition of HDACs or activation of HATs (increased CBP levels) may lead to increased expression of NPY in the amygdala that may be operative in the anxiolytic effects of acute ethanol exposure in rats. However, ethanol withdrawal-related anxiety might be related to decreased histone acetylation caused by activation of HDACs or inhibition of HATs (decreased CBP levels), and may also be responsible for the observed decrease in NPY expression in the amygdala. TSA treatment during ethanol withdrawal attenuated anxiety-like behaviors and rescued the reductions in both histone acetylation and NPY expression in the CeA and MeA during ethanol withdrawal. Thus, HDAC-induced chromatin remodeling in the amygdala may be crucial in the dynamic process that occurs during the development of alcoholism.
Similar articles
- Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article. - Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours.
Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Sakharkar AJ, et al. Int J Neuropsychopharmacol. 2014 Aug;17(8):1207-20. doi: 10.1017/S1461145714000054. Epub 2014 Feb 17. Int J Neuropsychopharmacol. 2014. PMID: 24528596 Free PMC article. - Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol.
Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Sakharkar AJ, et al. Alcohol Clin Exp Res. 2012 Jan;36(1):61-71. doi: 10.1111/j.1530-0277.2011.01581.x. Epub 2011 Jul 25. Alcohol Clin Exp Res. 2012. PMID: 21790673 Free PMC article. - [Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].
Legastelois R, Jeanblanc J, Vilpoux C, Bourguet E, Naassila M. Legastelois R, et al. Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French. - Epigenetic basis of the dark side of alcohol addiction.
Pandey SC, Kyzar EJ, Zhang H. Pandey SC, et al. Neuropharmacology. 2017 Aug 1;122:74-84. doi: 10.1016/j.neuropharm.2017.02.002. Epub 2017 Feb 4. Neuropharmacology. 2017. PMID: 28174112 Free PMC article. Review.
Cited by
- Epigenetic mechanisms in cerebral ischemia.
Schweizer S, Meisel A, Märschenz S. Schweizer S, et al. J Cereb Blood Flow Metab. 2013 Sep;33(9):1335-46. doi: 10.1038/jcbfm.2013.93. Epub 2013 Jun 12. J Cereb Blood Flow Metab. 2013. PMID: 23756691 Free PMC article. Review. - 7,8-Dihydroxyflavone Alleviates Anxiety-Like Behavior Induced by Chronic Alcohol Exposure in Mice Involving Tropomyosin-Related Kinase B in the Amygdala.
Wang N, Liu X, Li XT, Li XX, Ma W, Xu YM, Liu Y, Gao Q, Yang T, Wang H, Peng Y, Zhu XF, Guan YZ. Wang N, et al. Mol Neurobiol. 2021 Jan;58(1):92-105. doi: 10.1007/s12035-020-02111-0. Epub 2020 Sep 7. Mol Neurobiol. 2021. PMID: 32895785 - Stable Histone Methylation Changes at Proteoglycan Network Genes Following Ethanol Exposure.
Gavin DP, Hashimoto JG, Lazar NH, Carbone L, Crabbe JC, Guizzetti M. Gavin DP, et al. Front Genet. 2018 Aug 30;9:346. doi: 10.3389/fgene.2018.00346. eCollection 2018. Front Genet. 2018. PMID: 30214456 Free PMC article. - Dynamic changes in gene expression and alternative splicing mediate the response to acute alcohol exposure in Drosophila melanogaster.
Signor S, Nuzhdin S. Signor S, et al. Heredity (Edinb). 2018 Oct;121(4):342-360. doi: 10.1038/s41437-018-0136-4. Epub 2018 Aug 24. Heredity (Edinb). 2018. PMID: 30143789 Free PMC article. - Does Gender Leave an Epigenetic Imprint on the Brain?
Cortes LR, Cisternas CD, Forger NG. Cortes LR, et al. Front Neurosci. 2019 Feb 27;13:173. doi: 10.3389/fnins.2019.00173. eCollection 2019. Front Neurosci. 2019. PMID: 30872999 Free PMC article.
References
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. - PubMed
- Camelo S, Iglesias AH, Hwang D, Due B, Ryu H, Smith K, Gray SG, Imitola J, Duran G, Assaf B, Langley B, Khoury SJ, Stephanopoulos G, De Girolami U, Ratan RR, Ferrante RJ, Dangond F. Transcriptional therapy with histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. - PubMed
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich JB, Aunis D, Zwiller J. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous