Genetic variation in human NPY expression affects stress response and emotion - PubMed (original) (raw)
. 2008 Apr 24;452(7190):997-1001.
doi: 10.1038/nature06858. Epub 2008 Apr 2.
Guanshan Zhu, Ahmad R Hariri, Mary-Anne Enoch, David Scott, Rajita Sinha, Matti Virkkunen, Deborah C Mash, Robert H Lipsky, Xian-Zhang Hu, Colin A Hodgkinson, Ke Xu, Beata Buzas, Qiaoping Yuan, Pei-Hong Shen, Robert E Ferrell, Stephen B Manuck, Sarah M Brown, Richard L Hauger, Christian S Stohler, Jon-Kar Zubieta, David Goldman
Affiliations
- PMID: 18385673
- PMCID: PMC2715959
- DOI: 10.1038/nature06858
Genetic variation in human NPY expression affects stress response and emotion
Zhifeng Zhou et al. Nature. 2008.
Abstract
Understanding inter-individual differences in stress response requires the explanation of genetic influences at multiple phenotypic levels, including complex behaviours and the metabolic responses of brain regions to emotional stimuli. Neuropeptide Y (NPY) is anxiolytic and its release is induced by stress. NPY is abundantly expressed in regions of the limbic system that are implicated in arousal and in the assignment of emotional valences to stimuli and memories. Here we show that haplotype-driven NPY expression predicts brain responses to emotional and stress challenges and also inversely correlates with trait anxiety. NPY haplotypes predicted levels of NPY messenger RNA in post-mortem brain and lymphoblasts, and levels of plasma NPY. Lower haplotype-driven NPY expression predicted higher emotion-induced activation of the amygdala, as well as diminished resiliency as assessed by pain/stress-induced activations of endogenous opioid neurotransmission in various brain regions. A single nucleotide polymorphism (SNP rs16147) located in the promoter region alters NPY expression in vitro and seems to account for more than half of the variation in expression in vivo. These convergent findings are consistent with the function of NPY as an anxiolytic peptide and help to explain inter-individual variation in resiliency to stress, a risk factor for many diseases.
Figures
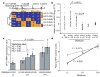
Figure 1. Haplotype-predicted NPY expression in brain, lymphoblasts and plasma
aConfiguration, frequencies (right) and cladistic clustering (left) of major NPY haplotypes (H1, H2, H3, H4 and H5; frequency more than 1%) in Finnish Caucasians (n = 516). b, Differential allele expression of NPY mRNA showing allelic ratio (log2(C/T)) of rs5574 in 28 heterozygous human post-mortem cerebella (Miami sample) grouped according to diplotypes (columns 2–5, means ± s.d.) to infer expression levels of H2, H3, H4 and H5 relative to H1 (the T allele is found only within H1, as shown in a). Diplotypes are compared using a two-tailed _t_-test. Column 1 presents all samples; column 6 shows controls for allelic amplification efficiency. c, Expression levels of the six common diplotypes in 47 lymphoblastoid cell lines derived from healthy Finnish Caucasians. Expression values of the three major haplotypes (H1, H2 and H3) were calculated from observed diplotype values (points and error bars show means ± s.e.m.) by multiple regression and were used to predict diplotype values (bars) under a co-dominant model (see Supplementary Fig. 2 for details, including fit to model). Diplotypes were grouped as low (LL), intermediate (LH) or high (HH) in expression. Numbers above columns are n. d, Diplotype-predicted plasma NPY levels (means ± s.e.m., standardized with Z scores; New Haven sample) in controls (black diamonds; LL, n = 16; LH, n = 113; HH, n = 39) and alcoholic patients (grey squares; LL, n = 29; LH, n = 72; HH, n = 27). P values were calculated by regression analysis.
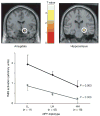
Figure 2. Effect of diplotype-predicted NPY mRNA expression on fMRI-measured amygdala and hippocampal activation in response to threat-related facial expressions
Top: statistical parametric maps representing NPY diplotype-biased (LL > LH > HH) mean right dorsal amygdala (x = 16, y = −8, z = −14, 64 voxels, t = 2.82, P = 0.003) and right hippocampal (x = 24, y = −20, z = −12, 132 voxels, t = 2.56, P = 0.006) activation is shown overlaid on an average sagittal and coronal MRI. Bottom: right amygdala (black diamonds) and hippocampal (grey squares) activities (means and s.e.m.) from clusters grouped by NPY diplotypes.
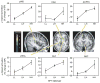
Figure 3. Effect of diplotype-predicted NPY mRNA expression on pain/stress-induced μ-opioid system activation
Activation (means ± s.e.m.) measured by the percentage change in the μ-opioid receptor binding potential (BP) among the NPY diplotypes (LL, n = 8; LH, n = 21; HH, n = 6) are shown in the dorsomedial prefrontal (dmPFC) and posterior insular (pINS) cortices, ventrolateral thalamus (vlTHA), ventral putamen (PUT) and caudate (CAU) nuclei, nucleus accumbens (NAC) and ventral amygdala (AMY; Supplementary Table 1). Z scores represented by the pseudocolour scale (left) are superimposed on an anatomically standardized MRI image. See also Supplementary Table 1 for details of the localization and statistics.
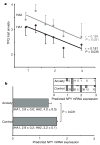
Figure 4. Correlations of diplotype-predicted NPY mRNA expression with TPQ trait anxiety and anxiety disorders in Finnish Caucasians
a, Bivariate regression of HA1 and HA2 scores (means and s.e.m.) and diplotype-predicted expression values in 137 healthy subjects (left to right: H1/H1, n = 33; H1/H3, n = 35; H3/H3, n = 5; H1/H2, n = 39; H2/H3, n = 15; H2/H2, n = 10). b, Diplotype-predicted NPY mRNA levels (means and s.e.m.) of n = 137 healthy controls and n = 18 anxiety patients by a two-tailed _t_-test (means ± s.e.m. for HA1 and HA2 are also shown). At the top right is a histogram comparing the numbers of controls and anxiety patients within each of the six diplotypes.
Comment in
- Is there an association between NPY and neuroticism?
Cotton CH, Flint J, Campbell TG. Cotton CH, et al. Nature. 2009 Apr 2;458(7238):E6; discussion E7. doi: 10.1038/nature07927. Nature. 2009. PMID: 19340021
Similar articles
- Emotion processing, major depression, and functional genetic variation of neuropeptide Y.
Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT, Langenecker SA, Love TM, Peciña M, Shafir T, Stohler CS, Goldman D, Zubieta JK. Mickey BJ, et al. Arch Gen Psychiatry. 2011 Feb;68(2):158-66. doi: 10.1001/archgenpsychiatry.2010.197. Arch Gen Psychiatry. 2011. PMID: 21300944 Free PMC article. - Genetic modulation of plasma NPY stress response is suppressed in substance abuse: association with clinical outcomes.
Xu K, Hong KA, Zhou Z, Hauger RL, Goldman D, Sinha R. Xu K, et al. Psychoneuroendocrinology. 2012 Apr;37(4):554-64. doi: 10.1016/j.psyneuen.2011.08.005. Epub 2011 Sep 13. Psychoneuroendocrinology. 2012. PMID: 21917383 Free PMC article. - Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression.
Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, Zwanzger P, Heindel W, Deckert J, Arolt V, Suslow T, Baune BT. Domschke K, et al. Eur Neuropsychopharmacol. 2010 May;20(5):301-9. doi: 10.1016/j.euroneuro.2009.09.006. Epub 2009 Oct 24. Eur Neuropsychopharmacol. 2010. PMID: 19854625 - Brain neuropeptide Y (NPY) in stress and alcohol dependence.
Heilig M, Thorsell A. Heilig M, et al. Rev Neurosci. 2002;13(1):85-94. doi: 10.1515/revneuro.2002.13.1.85. Rev Neurosci. 2002. PMID: 12013027 Review. - Neuropeptide Y and posttraumatic stress disorder.
Sah R, Geracioti TD. Sah R, et al. Mol Psychiatry. 2013 Jun;18(6):646-55. doi: 10.1038/mp.2012.101. Epub 2012 Jul 17. Mol Psychiatry. 2013. PMID: 22801411 Free PMC article. Review.
Cited by
- Stress and substance use disorders: risk, relapse, and treatment outcomes.
Sinha R. Sinha R. J Clin Invest. 2024 Aug 15;134(16):e172883. doi: 10.1172/JCI172883. J Clin Invest. 2024. PMID: 39145454 Free PMC article. Review. - The role of resilience in the relationship between stress and alcohol.
Schwandt ML, Cullins E, Ramchandani VA. Schwandt ML, et al. Neurobiol Stress. 2024 May 16;31:100644. doi: 10.1016/j.ynstr.2024.100644. eCollection 2024 Jul. Neurobiol Stress. 2024. PMID: 38827175 Free PMC article. - Pain-related single nucleotide polymorphisms: association with lumbar spinal stenosis patient experience and non-surgical treatment outcomes.
Ernst S, Huang W, Conley Y, Vo N, Schneider M, Sowa G. Ernst S, et al. Eur Spine J. 2024 Jun;33(6):2213-2221. doi: 10.1007/s00586-024-08182-0. Epub 2024 Apr 6. Eur Spine J. 2024. PMID: 38581434 - The missing hallmark of health: psychosocial adaptation.
López-Otín C, Kroemer G. López-Otín C, et al. Cell Stress. 2024 Mar 12;8:21-50. doi: 10.15698/cst2024.03.294. eCollection 2024. Cell Stress. 2024. PMID: 38476764 Free PMC article. - The effects of Korean Red Ginseng on stress-related neurotransmitters and gene expression: A randomized, double-blind, placebo-controlled trial.
Yoon J, Park B, Hong KW, Jung DH. Yoon J, et al. J Ginseng Res. 2023 Nov;47(6):766-772. doi: 10.1016/j.jgr.2023.08.001. Epub 2023 Aug 12. J Ginseng Res. 2023. PMID: 38107397 Free PMC article.
References
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–222. - PubMed
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. - PubMed
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. - PubMed
- Adrian TE, et al. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. - PubMed
- Allen YS, et al. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL040962/HL/NHLBI NIH HHS/United States
- R01-AA13892/AA/NIAAA NIH HHS/United States
- R01 DE015396/DE/NIDCR NIH HHS/United States
- R01 DA 016423/DA/NIDA NIH HHS/United States
- R01 MH074697/MH/NIMH NIH HHS/United States
- Z01 AA000301-09/ImNIH/Intramural NIH HHS/United States
- P50 DA016556/DA/NIDA NIH HHS/United States
- R01 MH074697-04A1/MH/NIMH NIH HHS/United States
- K02 DA017232/DA/NIDA NIH HHS/United States
- R01 HL065137/HL/NHLBI NIH HHS/United States
- R01 DE 15396/DE/NIDCR NIH HHS/United States
- PL1 DA024859-02/DA/NIDA NIH HHS/United States
- R01 AA013892/AA/NIAAA NIH HHS/United States
- Z99 AA999999/ImNIH/Intramural NIH HHS/United States
- R01 DA016423/DA/NIDA NIH HHS/United States
- K02-DA17232/DA/NIDA NIH HHS/United States
- PL1 DA024859/DA/NIDA NIH HHS/United States
- P50-DA16556/DA/NIDA NIH HHS/United States
- K01 MH072837/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous