TRPA1 is a major oxidant sensor in murine airway sensory neurons - PubMed (original) (raw)
TRPA1 is a major oxidant sensor in murine airway sensory neurons
Bret F Bessac et al. J Clin Invest. 2008 May.
Abstract
Sensory neurons in the airways are finely tuned to respond to reactive chemicals threatening airway function and integrity. Nasal trigeminal nerve endings are particularly sensitive to oxidants formed in polluted air and during oxidative stress as well as to chlorine, which is frequently released in industrial and domestic accidents. Oxidant activation of airway neurons induces respiratory depression, nasal obstruction, sneezing, cough, and pain. While normally protective, chemosensory airway reflexes can provoke severe complications in patients affected by inflammatory airway conditions like rhinitis and asthma. Here, we showed that both hypochlorite, the oxidizing mediator of chlorine, and hydrogen peroxide, a reactive oxygen species, activated Ca(2+) influx and membrane currents in an oxidant-sensitive subpopulation of chemosensory neurons. These responses were absent in neurons from mice lacking TRPA1, an ion channel of the transient receptor potential (TRP) gene family. TRPA1 channels were strongly activated by hypochlorite and hydrogen peroxide in primary sensory neurons and heterologous cells. In tests of respiratory function, Trpa1(-/-) mice displayed profound deficiencies in hypochlorite- and hydrogen peroxide-induced respiratory depression as well as decreased oxidant-induced pain behavior. Our results indicate that TRPA1 is an oxidant sensor in sensory neurons, initiating neuronal excitation and subsequent physiological responses in vitro and in vivo.
Figures
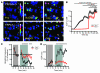
Figure 1. NaOCl induces Ca2+ influx and ionic currents in mustard oil–responsive sensory neurons.
(A) Activation of Ca2+ influx into cultured murine DRG, nodose (ND), and trigeminal (TG) neurons by NaOCl, as measured by fluorescent Fura-2 imaging, before and 70 s after challenge with NaOCl (24 ppm), followed by 100 μM mustard oil (MO) after 40 s. Pseudocolors denote 0–3 μM [Ca2+]i. Original magnification, ×10. (B) [Ca2+]i concentration of DRG neurons activated by application of NaOCl followed by mustard oil, capsaicin (Cap), and 65 mM KCl. Thick and thin lines denote mean and ± SEM, respectively. Neurons (n = 300) were analyzed from 4 mice at ×10 magnification. (C) Kinetics of OCl–-activated cationic currents and ruthenium red–induced block in cultured murine DRG neurons. NaOCl was superfused after 20 s; after 60 s, ruthenium red (RuRed) was added to the NaOCl. Currents were measured using a ±80 mV voltage ramp protocol over 100 ms at 0.5-Hz intervals (0 mV holding potential throughout). Black and gray lines denote mean and ± SEM, respectively, of currents from 6 neurons at –80 and +80 mV plotted versus time. Intracellular Cs-based solution contained 10 mM EGTA. (D) Representative current-voltage relationship of a OCl–-sensitive DRG neuron before application of NaOCl (black), during maximal activation by NaOCl (24 ppm, green), and after application of 10 μM ruthenium red (red). Residual voltage gated currents observed are caused by incomplete inactivation of voltage-gated channels. Currents were measured as in C.
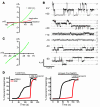
Figure 2. NaOCl activation of cloned TRPA1 channels in HEK293t cells.
(A) Activation of Ca2+ influx into hTRPA1-transfected HEK293t cells by NaOCl (24 ppm) and 100 μM mustard oil. Pre, unstimulated. Pseudocolors denote 0–3 μM [Ca2+]i. (B) Dose-response relationship of NaOCl-activated Ca2+ influx into cultured DRG neurons (black; n = 25 ± 1 cells/dose), mTRPA1-expressing (green; n = 23 ± 5 cells/dose), and hTRPA1-expressing HEK293t cells (red; n = 38 ± 8 cells/dose). Cells were activated with the indicated concentrations of NaOCl, then with 100 μM mustard oil. Baseline [Ca2+]i level was subtracted for each cell. Error bars denote SEM. (C) Kinetics of NaOCl-induced currents through hTRPA1 channels expressed in HEK293t cells. NaOCl was added to the bath solution after 40 s. Currents were measured using a ±80 mV voltage ramp protocol over 100 ms at 0.5-Hz intervals (0 mV holding potential throughout). Black and gray lines denote mean and ± SEM, respectively, of current amplitudes at –80 and +80 mV plotted versus time (n = 4). Intracellular Cs-based solution contained 10 mM EGTA. (D) Current-voltage relationship of a representative hTRPA1-transfected HEK293t cell before activation (black), during maximal NaOCl activation (24 ppm; green), and at the end of the inactivation phase (red). (E) Chemical structure of chloramine-T. (F) Activation of Ca2+ influx into hTRPA1-transfected HEK293t cells by 100 μM chloramine-T and 100 μM mustard oil. Pseudocolors denote 0–3 μM [Ca2+]i.
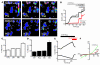
Figure 3. Lack of NaOCl-induced Ca2+ influx in sensory neurons and respiratory insensitivity to NaOCl aerosol in Trpa1–/– mice.
(A) Responses of cultured DRG neurons from littermate Trpa1+/+ and Trpa1–/– mice to NaOCl (24 ppm) followed by 5 μM capsaicin, as measured by Fura-2 imaging. Trpa1–/– neurons showed no [Ca2+]i increase after NaOCl exposure, but were activated by capsaicin. Pseudocolors denote 0–3 μM [Ca2+]i. (B) Activation of Ca2+ influx into DRG neurons plotted against time. Average [Ca2+]i concentration of neurons activated by application of NaOCl followed by mustard oil, capsaicin, and 65 mM KCl. Thick and thin lines denote mean and ± SEM, respectively. Neurons (n = 300 [_Trpa1+/+_]; 263 [_Trpa1–/–_]) cultured from 4 mice per group were analyzed at ×10 magnification. (C) Effects of exposure to NaOCl aerosol on respiratory frequencies, as measured by unrestrained plethysmography. Mice were exposed to vehicle aerosol (10 min), room air flush (2 min), NaOCl aerosol (15 min), and then air (8 min). Values denote percentage of baseline (initial vehicle exposure). Respiratory frequency was slightly affected in Trpa1–/– mice, but dramatically declined in Trpa1+/+ mice during NaOCl exposure and when NaOCl aerosol was replaced by air. Error bars denote SEM. P < 0.00005 between groups; P < 0.000001 over time (repeated-measures ANOVA). n = 11 per group. (D) EEP duration during exposure to NaOCl aerosol. Data were collected from the experiment described in C. Unlike Trpa1–/– mice, Trpa1+/+ mice responded to NaOCl aerosol with an approximately 3-fold increase in EEP duration, which remained elevated 8 min after the end of NaOCl exposure. P < 0.000001 between groups, over time, and for the interaction of genotype and time (repeated-measures ANOVA). Single asterisks denote significant differences (Bonferroni post-hoc analysis; α = 0.05) for individual time points.
Figure 5. Activation of heterologously expressed TRPA1 channels by H2O2.
(A) Representative whole-cell current-voltage relationship of hTRPA1 currents in CHO cells before activation (black), during maximal activation by H2O2 (green), and at the end of the inactivation phase (red). Currents were measured using a ±80 mV voltage ramp protocol over 100 ms at 0.5-Hz intervals (0 mV holding potential throughout). Intracellular Cs-based solution contained 10 mM EGTA. (B) Single TRPA1 channel currents induced by H2O2 in the cell-attached configuration. Cell-attached patches were formed on CHO cells expressing hTRPA1. The cell was superfused with a bath solution containing 1 mM H2O2, and single-channel openings were recorded using a ±60 mV step protocol. Membrane potential was estimated to be –10 mV, where zero currents were observed. Indicated potentials are corrected values. Representative current traces from a patch containing 3 channels are shown. hTRPA1 unitary conductance was 42 ± 0.5 pS (–50 mV) and 73 ± 1.0 pS (+50 mV). Pipette and bath solutions contained 2 mM Ca2+. (C) Current-voltage relationship of open channel conductance of single hTRPA1 channels recorded in CHO cells in cell-attached configuration. Values are mean ± SEM of 10 channel openings each. Potentials were corrected as in B. (D) Requirement of covalent agonist acceptor sites for TRPA1 activation by NaOCl and H2O2. [Ca2+]i changes were compared between HEK293t cells expressing hTRPA1 WT channels and cells expressing TRPA1 channels with mutated interaction sites (C619, C639, C663, and K708; denoted 3CK). A 1-mM dose of nonreactive agonist carvacrol was given after the indicated oxidant stimulus. Values denote percent maximal response to carvacrol (n = 60 cells/trace).
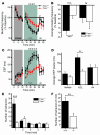
Figure 4. H2O2 induces TRPA1-dependent influx of Ca2+ and ionic currents in mustard oil–responsive sensory neurons.
(A) Responses of cultured DRG neurons from littermate Trpa1+/+ and Trpa1–/– mice to 5 mM H2O2, followed by 3 μM capsaicin, as measured by Fura-2 imaging. Trpa1–/– neurons showed no [Ca2+]i increase after H2O2 exposure, but were activated by capsaicin. Pseudocolors denote 0–3 μM [Ca2+]i. (B) Activation of Ca2+ influx by H2O2 into DRG neurons plotted against time. Average [Ca2+]i concentration of neurons activated by application of H2O2 followed by mustard oil, capsaicin, and 65 mM KCl. Thick and thin lines denote mean and ± SEM, respectively. Neurons (n = 189 [_Trpa1+/+_]; 146 [_Trpa1–/–_]) were analyzed at ×10 magnification. (C) Activation of DRG neurons (n = 161) by 5 mM H2O2, NaOCl (24 ppm), 100 μM mustard oil, 5 μM capsaicin, and 65 mM KCl. Neurons were considered activated when [Ca2+]i exceeded 500 nM. Values denote activated KCl-sensitive cells. (D) Activation of DRG neurons (n = 130) by NaOCl (24 ppm), 5 mM H2O2, 100 μM mustard oil, 5 μM capsaicin, and 65 mM KCl. (E) Kinetics of H2O2-activated cationic currents and ruthenium red–induced block in a cultured murine sensory neuron. H2O2 was superfused after 50-s initiation of whole-cell configuration, after which ruthenium red was coapplied at 210 s. Currents were measured using a ±80 mV voltage ramp protocol over 100 ms at 0.5-Hz intervals (0 mV holding potential throughout). Intracellular Cs-based solution contained 10 mM EGTA. (F) Representative current-voltage relationships of currents recorded from a DRG neuron before application of H2O2 (black), during maximal activation by H2O2 (green), and after application of 20 μM ruthenium red (red). Currents were measured as in C. Error bars represent SEM.
Figure 6. Reduced respiratory and nociceptive sensitivity of Trpa1–/– mice to H2O2.
(A) Change in respiratory frequency during aerosol exposure to PBS and 3% H2O2, as measured by unrestrained plethysmography. Mice were exposed to vehicle aerosol (10 min), room air flush (2 min), H2O2 aerosol (15 min), and then air (8 min). Values denote percent baseline (initial vehicle exposure). Both groups showed a significant decrease in respiratory frequency during H2O2 exposure compared with vehicle; however, the reduction in Trpa1+/+ mice was significantly greater. P < 0.005 between groups; P < 0.000001 over time (repeated-measures ANOVA). (B) Changes in respiratory frequency after exposure to H2O2 (2–15 min) and air (8 min) as in A. Values denote percent baseline (initial vehicle exposure). (C) Changes in EEP duration during exposure to H2O2 aerosol as in A. P < 0.001 between groups; P < 0.000001 over time (repeated-measures ANOVA). Single asterisks denote significant differences (Bonferroni post-hoc analysis; α = 0.05) for individual time points in A and C. (D) Change in EEP over duration of exposure to vehicle (2–10 min), H2O2 (2–15 min), and air (8 min). (E) Nocifensive responses following paw injection of 25 μl of 0.1% H2O2 solution (32 mM). The number of nocifensive responses (paw flicks, licks, and lifts) was averaged in 1-min intervals. (F) Pain responses accumulated over a 5-min period following injection of H2O2 as in E. (A–D) n = 14 per group. (E and F) n = 12 (Trpa1+/+), 11 (Trpa1–/–). Error bars denote SEM. **P < 0.01; ***P < 0.001 (ANOVA).
Similar articles
- Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation.
Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Akopian AN, et al. J Neurosci. 2008 Jan 30;28(5):1064-75. doi: 10.1523/JNEUROSCI.1565-06.2008. J Neurosci. 2008. PMID: 18234885 Free PMC article. - Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels.
Nesuashvili L, Hadley SH, Bahia PK, Taylor-Clark TE. Nesuashvili L, et al. Mol Pharmacol. 2013 May;83(5):1007-19. doi: 10.1124/mol.112.084319. Epub 2013 Feb 26. Mol Pharmacol. 2013. PMID: 23444014 Free PMC article. - A TRPA1-dependent mechanism for the pungent sensation of weak acids.
Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. Wang YY, et al. J Gen Physiol. 2011 Jun;137(6):493-505. doi: 10.1085/jgp.201110615. Epub 2011 May 16. J Gen Physiol. 2011. PMID: 21576376 Free PMC article. - Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures.
Bessac BF, Jordt SE. Bessac BF, et al. Proc Am Thorac Soc. 2010 Jul;7(4):269-77. doi: 10.1513/pats.201001-004SM. Proc Am Thorac Soc. 2010. PMID: 20601631 Free PMC article. Review. - Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control.
Bessac BF, Jordt SE. Bessac BF, et al. Physiology (Bethesda). 2008 Dec;23:360-70. doi: 10.1152/physiol.00026.2008. Physiology (Bethesda). 2008. PMID: 19074743 Free PMC article. Review.
Cited by
- TRP channels as sensors of oxygen availability.
Numata T, Ogawa N, Takahashi N, Mori Y. Numata T, et al. Pflugers Arch. 2013 Aug;465(8):1075-85. doi: 10.1007/s00424-013-1237-9. Epub 2013 Feb 17. Pflugers Arch. 2013. PMID: 23417605 Review. - TRPA1 as a drug target--promise and challenges.
Chen J, Hackos DH. Chen J, et al. Naunyn Schmiedebergs Arch Pharmacol. 2015 Apr;388(4):451-63. doi: 10.1007/s00210-015-1088-3. Epub 2015 Feb 3. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25640188 Free PMC article. Review. - Neural Abnormalities in Nonallergic Rhinitis.
Bernstein JA, Singh U. Bernstein JA, et al. Curr Allergy Asthma Rep. 2015 Apr;15(4):18. doi: 10.1007/s11882-015-0511-7. Curr Allergy Asthma Rep. 2015. PMID: 26130469 Review. - Transient Receptor Potential Ankyrin 1 (TRPA1)-An Inflammation-Induced Factor in Human HaCaT Keratinocytes.
Luostarinen S, Hämäläinen M, Moilanen E. Luostarinen S, et al. Int J Mol Sci. 2021 Mar 24;22(7):3322. doi: 10.3390/ijms22073322. Int J Mol Sci. 2021. PMID: 33805042 Free PMC article. - Involvement of the gut chemosensory system in the regulation of colonic anion secretion.
Kuwahara A. Kuwahara A. Biomed Res Int. 2015;2015:403919. doi: 10.1155/2015/403919. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866781 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous