Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid - PubMed (original) (raw)
Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid
Kathryn E Nichol et al. J Neuroinflammation. 2008.
Abstract
Background: Inflammation is associated with Abeta pathology in Alzheimer's disease (AD) and transgenic AD models. Previously, it has been demonstrated that chronic stimulation of the immune response induces pro-inflammatory cytokines IL-1beta and TNF-alpha which contribute to neurodegeneration. However, recent evidence has shown that inducing the adaptive immune response reduces Abeta pathology and is neuroprotective. Low concentrations of IFN-gamma modulate the adaptive immune response by directing microglia to differentiate to antigen presenting cells. Our objective was to determine if exercise could induce a shift from the immune profile in aged (17-19 months) Tg2576 mice to a response that reduces Abeta pathology.
Methods: TG (n = 29) and WT (n = 27) mice were divided into sedentary (SED) and exercised (RUN) groups. RUN animals were provided an in-cage running wheel for 3 weeks. Tissue was harvested and hippocampus and cortex dissected out. Quantitative data was analyzed using 2 x 2 ANOVA and student's t-tests.
Results: IL-1beta and TNF-alpha were significantly greater in hippocampi from sedentary Tg2576 (TGSED) mice than in wildtype (WTSED) (p = 0.04, p = 0.006). Immune response proteins IFN-gamma and MIP-1alpha are lower in TGSED mice than in WTSED (p = 0.03, p = 0.07). Following three weeks of voluntary wheel running, IL-1beta and TNF-alpha decreased to levels indistinguishable from WT. Concurrently, IFN-gamma and MIP-1alpha increased in TGRUN. Increased CD40 and MHCII, markers of antigen presentation, were observed in TGRUN animals compared to TGSED, as well as CD11c staining in and around plaques and vasculature. Additional vascular reactivity observed in TGRUN is consistent with an alternative activation immune pathway, involving perivascular macrophages. Significant decreases in soluble Abeta40 (p = 0.01) and soluble fibrillar Abeta (p = 0.01) were observed in the exercised transgenic animals.
Conclusion: Exercise shifts the immune response from innate to an adaptive or alternative response. This shift in immune response coincides with a decrease in Abeta in advanced pathological states.
Figures
Figure 1
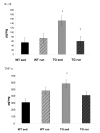
IL-1β is significantly greater in sedentary Tg2576 mice than in WT sedentary mice (p = 0.006). Exercise results in a significantly lower level of IL-1β in the Tg2576 (p = 0.01). The level of IL-1β in exercised Tg2576 mice (TGRUN) is no longer distingushable from the WT mouse (WTSED). TNF-α is significantly greater in sedentary Tg2576 mice (TG sed) than in WT sedentary mice (p = 0.04). Exercise reduces TNF-α in TG mice (TG run) to a level indistinguishable from the WT (WTSED). *Significantly different from sedentary WT † significantly different from sedentary Tg2576.
Figure 2
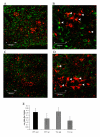
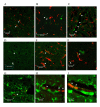
CD11b positive microglia (green immunofluorescence) in TGSED(A). Higher magnification reveals some co-labeling with microglial marker Iba-1 (red) (B, arrowheads). CD11b positive glia are present in TGRUN (C) and co-labeled with Iba-1 (red) in some cases (D, arrowheads). Overall levels of Iba-1 (normalized to actin) are not significantly different based on condition or genotype (E). High immunoreactivity for Iba-1 in WT is likely due to the advanced age of the animals used.
Figure 3
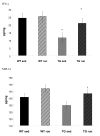
IFN-γ is significantly lower in the Tg2576 sedentary mice that in the WT sedentary mice (p = 0.03). Exercise resulted in increased levels of IFN-γ in the Tg2576 mouse (TGRUN) to a level indistinguishable form the WT (WT). MIP-1α demonstrated a trend of being lower in TGSED compared to the WT (p = 0.07), but was significantly increased by exercise (TGRUN) (p = 0.05). *Significantly different from sedentary WT; † significantly different from sedentary Tg2576.
Figure 4
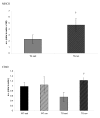
MHC II levels were significantly greater in TGRUN than TGSED (p = 0.04). CD40 is significantly greater in TGRUN compared to TGSED (p = 0.008). WTSED tended to have greater levels of CD40 than TGSED, but this difference failed to achieve significance (p = 0.10). † Significantly different from sedentary Tg2576.
Figure 5
CD11c positive microglia (green immunofluorescence) are present in TGSED and colocalize with Iba-1 (arrowheads) but do not appear vascular (A). CD11c labeling in TGRUN appeared in cells not labeled by Iba-1 (red) that were linearly arranged, perhaps within or around microvessels. (B, D). Larger vessels had CD11c labeling along the vessel wall, perhaps in the perivascular space (C). Using macrophage markers CD68, we observed microvascular labeling again only in TGRUN (D). Double labeling for CD11c (green) and CD68 (red) revealed that CD11c+ cells were adjacent to CD68+ cells in and around vasculature (arrows) (E, F). Using mannose receptor antibody (red), specific for perivascular macrophages, we again observed vascular labeling only in TGRUN (G-I). High magnification shows the mannose receptor labeled cells are within vessels (H, I)(arrowheads). Green indicates Iba-1 labeling for microglia in and around vessels (G-I).
Figure 6
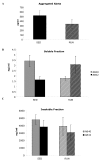
Aβ analysis by multiplex and ELISA. Aggregated Aβ levels are not significantly lower in hippocampus of TGRUN compared to TGSED, though a 35% decrease is observed in means. (A) Aβ40 but not Aβ42 is significantly lower in soluble fractions from cortex of TGRUN and TGSED (p = 0.01)(B). There are no significant differences in insoluble fractions (C).
Figure 7
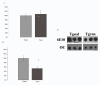
Aβ analysis by dot blot. No differences existed between TGSED and TGRUN for total Aβ in the soluble fraction of hippocampal samples, evaluated by 6E10 antibody (A). Aβ fibrils, detected by OC antibody, were significantly decreased in TGRUN animals compared to TGSED (p = 0.01)(B). A representative dot blot is shown (C).
Similar articles
- Amyloid Beta Pathology Exacerbates Weight Loss and Brain Cytokine Responses following Low-Dose Lipopolysaccharide in Aged Female Tg2576 Mice.
Knopp RC, Baumann KK, Wilson ML, Banks WA, Erickson MA. Knopp RC, et al. Int J Mol Sci. 2022 Feb 21;23(4):2377. doi: 10.3390/ijms23042377. Int J Mol Sci. 2022. PMID: 35216491 Free PMC article. - Age-dependent neuroplasticity mechanisms in Alzheimer Tg2576 mice following modulation of brain amyloid-β levels.
Lilja AM, Röjdner J, Mustafiz T, Thomé CM, Storelli E, Gonzalez D, Unger-Lithner C, Greig NH, Nordberg A, Marutle A. Lilja AM, et al. PLoS One. 2013;8(3):e58752. doi: 10.1371/journal.pone.0058752. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554921 Free PMC article. - Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease.
Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, Timson BF, Csernansky JG. Yuede CM, et al. Neurobiol Dis. 2009 Sep;35(3):426-32. doi: 10.1016/j.nbd.2009.06.002. Epub 2009 Jun 12. Neurobiol Dis. 2009. PMID: 19524672 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Inflammaging as a prodrome to Alzheimer's disease.
Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J. Giunta B, et al. J Neuroinflammation. 2008 Nov 11;5:51. doi: 10.1186/1742-2094-5-51. J Neuroinflammation. 2008. PMID: 19014446 Free PMC article. Review.
Cited by
- Physical exercise in the prevention and treatment of Alzheimer's disease.
De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, Millan F, Salvador-Pascual A, García-Lucerga C, Blasco-Lafarga C, Garcia-Dominguez E, Carretero A, Correas AG, Viña J, Gomez-Cabrera MC. De la Rosa A, et al. J Sport Health Sci. 2020 Sep;9(5):394-404. doi: 10.1016/j.jshs.2020.01.004. Epub 2020 Feb 4. J Sport Health Sci. 2020. PMID: 32780691 Free PMC article. Review. - Treating depression and depression-like behavior with physical activity: an immune perspective.
Eyre HA, Papps E, Baune BT. Eyre HA, et al. Front Psychiatry. 2013 Feb 4;4:3. doi: 10.3389/fpsyt.2013.00003. eCollection 2013. Front Psychiatry. 2013. PMID: 23382717 Free PMC article. - The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging.
Vecchio LM, Meng Y, Xhima K, Lipsman N, Hamani C, Aubert I. Vecchio LM, et al. Brain Plast. 2018 Dec 12;4(1):17-52. doi: 10.3233/BPL-180069. Brain Plast. 2018. PMID: 30564545 Free PMC article. Review. - Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging.
Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Ahlskog JE, et al. Mayo Clin Proc. 2011 Sep;86(9):876-84. doi: 10.4065/mcp.2011.0252. Mayo Clin Proc. 2011. PMID: 21878600 Free PMC article. Review. - The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade.
Cline EN, Bicca MA, Viola KL, Klein WL. Cline EN, et al. J Alzheimers Dis. 2018;64(s1):S567-S610. doi: 10.3233/JAD-179941. J Alzheimers Dis. 2018. PMID: 29843241 Free PMC article. Review.
References
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. - PubMed
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous