Efficient retroviral transduction of human B-lymphoid and myeloid progenitors: marked inhibition of their growth by the Pax5 transgene - PubMed (original) (raw)
Efficient retroviral transduction of human B-lymphoid and myeloid progenitors: marked inhibition of their growth by the Pax5 transgene
Rieko Sekine et al. Int J Hematol. 2008 May.
Abstract
We applied a coculture system for the genetic manipulation of human B-lymphoid and myeloid progenitor cells using murine bone marrow stromal cell support, and investigated the effects of forced Pax5 expression in both cell types. Cytokine-stimulated cord blood CD34+ cells could be transduced at 85% efficiency and 95% cell viability by a single 24-h infection with RD114-pseudotyped retroviral vectors, produced by the packaging cell line Plat-F and bicistronic vector plasmids pMXs-Ig, pMYs-Ig, or pMCs-Ig, encoding EGFP. Infected CD34+ cells were seeded onto HESS-5 cells in the presence of stem cell factor and granulocyte colony-stimulating factor, allowing the extensive production of B progenitors and granulocytic cells. We examined the cell number and CD34, CD33, CD19, and CD20 lambda and kappa expressions by flow cytometry. Ectopic expression of Pax5 in CD34+ cells resulted in small myeloid progenitors coexpressing CD33 and CD19 and inhibited myeloid differentiation. After 6 weeks, the number of Pax5-transduced CD19+ cells was 40-fold lower than that of control cells. However, the expression of CD20 and the kappa/lambda chain on Pax5-transduced CD19+ cells suggests that the Pax5 transgene may not interfere with their differentiation. This report is the first to describe the effects of forced Pax5 expression in human hematopoietic progenitors.
Figures
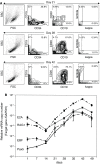
Fig. 1
a Sequential phenotypic analysis of in vitro B-cell differentiation examined by flow cytometry of CD34, CD19, CD20, and light chain (kappa or lambda) expression. The quadrate gate of two-color analysis was determined by each isotype control. One representative result of four repeat experiments is shown. b Kinetic analysis of B-lymphopoiesis-associated gene expression by QR-PCR for the transcription factors E2A, EBF, Pax5, and RAG1 proteins. The results are the mean ± SD of duplicate experiments
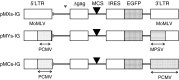
Fig. 2
Structure of pMXs-Ig, pMYs-Ig, and pMCs-Ig retrovirus vectors. ψ packaging signal, Δ gag truncated gag sequence, LTR long terminal repeat, MCS multi-cloning site, IRES internal ribosomal entry site, EGFP enhanced green fluorescent protein, white box Mo-MLV LTR, gray box PCMV LTR, hatched box MPSV LTR
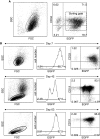
Fig. 3
a The relationship between EGFP expression and the surface marker CD34 at the end of infection. Cells were stained with PE-conjugated anti-CD34 and analyzed by two-color FCM. b After a 4-day coculture, EGFP expression levels in CD33+ cells were compared between the three vectors by FCM, and cytospin preparations revealed an immature myeloid cell morphology. The photograph of pMCs-Ig cells shown is representative (×600). c Coculture at 28-days. Constitutive EGFP expression by pMXs, pMYs, and pMCs in mature myeloid cells (upper panels), and B-lymphoid cells (lower panels). The photograph of pMCs-Ig cells is representative (×600)
Fig. 4
a Transduction rates evaluated by the percentage of EGFP+ cells and percentage of CD34+ cells after 4× repeat infection. The mean transduction rate and SD were 80.4 ± 6%, the mean percentage of CD34+ cells decreased to 75.8 ± 2.7%, and the percentage of EGFP+ cells in the CD34-positive gate was 86.3 ± 4.6% (n = 3). The data shown are from one representative experiment of three. b The stability of EGFP expression in pMCs-Ig-transduced cells was determined after 1, 6, and 9 weeks of coculture. Non-adherent cells in the culture medium on day 7 (upper). Almost all cells were developing into CD33+ myeloid cells. All of the cocultured cells were trypsinized on day 42 (middle) and day 63 (lower), developing into CD19+ B-lymphoid cells. In each case, the percentage of EGFP+ cells determined by FACS was above 98%
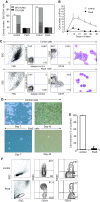
Fig. 5
a White box total number of control colonies, black box total number of Pax5 colonies, slanted box BFU-E colony plus mixed colony numbers, gray box CFU-GM colony number. Three hundred control and Pax5 cells were seeded, and the colony number was counted 14 days later (the data shown are representative of four independent experiments). b The total weekly number of non-adherent cells, control cells (open circles) versus Pax5 cells (filled circles). The number of Pax5 cells was consistently lower than that of control cells. c Surface marker and morphology of myeloid cells after 1 week of coculture. EGFP+ myeloid cells were stained with PE-CD33 and APC-CD19 and examined by three-color FCM. A minor but significant fraction of non-adherent cells could be detected in the lymphoid gate of the cell population transduced with Pax5 vector, but not the control vector. M-G-stained cytospin preparations revealed the myelogenetic maturation arrest of Pax5 cells (×600). One representative result of three repeat experiments is shown. d Image capture with an inverted Nikon TMD300 microscope after 1 and 6 weeks of coculture (×400). The cobblestone area generated by Pax5 cells was markedly reduced. e Total cell number of control and Pax5 cells on day 42 (n = 3). f Living EGFP+ cells from the Pax5-transduced CD34+ cells expressed CD20 at a higher level than the control. A small number of EGFP+ cells also expressed the kappa light chain (these data are representative of three independent experiments)
Fig. 6
a Cell cycle analysis using 70% ethanol fixation and the apoptosis assay, comparing Pax5 cells with control cells on days 7, 14, and 21. b Cell cycle analysis of EGFP-positive cells using formaldehyde fixation and the apoptosis assay, comparing Pax5 cells with control cells on day 28
Similar articles
- Biphenotypic B-lymphoid/myeloid cells expressing low levels of Pax5: potential targets of BAL development.
Simmons S, Knoll M, Drewell C, Wolf I, Mollenkopf HJ, Bouquet C, Melchers F. Simmons S, et al. Blood. 2012 Nov 1;120(18):3688-98. doi: 10.1182/blood-2012-03-414821. Epub 2012 Aug 27. Blood. 2012. PMID: 22927250 - Ectopic expression of PAX5 promotes maintenance of biphenotypic myeloid progenitors coexpressing myeloid and B-cell lineage-associated genes.
Anderson K, Rusterholz C, Månsson R, Jensen CT, Bacos K, Zandi S, Sasaki Y, Nerlov C, Sigvardsson M, Jacobsen SE. Anderson K, et al. Blood. 2007 May 1;109(9):3697-705. doi: 10.1182/blood-2006-05-026021. Epub 2007 Jan 11. Blood. 2007. PMID: 17218387 - Ordering human CD34+CD10-CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways.
Sanz E, Muñoz-A N, Monserrat J, Van-Den-Rym A, Escoll P, Ranz I, Alvarez-Mon M, de-la-Hera A. Sanz E, et al. Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5925-30. doi: 10.1073/pnas.0907942107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231472 Free PMC article. - The Pleiotropy of PAX5 Gene Products and Function.
Nasri Nasrabadi P, Martin D, Gharib E, Robichaud GA. Nasri Nasrabadi P, et al. Int J Mol Sci. 2022 Sep 3;23(17):10095. doi: 10.3390/ijms231710095. Int J Mol Sci. 2022. PMID: 36077495 Free PMC article. Review. - Models of haematopoiesis: seeing the wood for the trees.
Ceredig R, Rolink AG, Brown G. Ceredig R, et al. Nat Rev Immunol. 2009 Apr;9(4):293-300. doi: 10.1038/nri2525. Nat Rev Immunol. 2009. PMID: 19282853 Review.
Cited by
- Bioimaging analysis of nuclear factor-κB activity in Philadelphia chromosome-positive acute lymphoblastic leukemia cells reveals its synergistic upregulation by tumor necrosis factor-α-stimulated changes to the microenvironment.
Tsai HJ, Kobayashi S, Izawa K, Ishida T, Watanabe T, Umezawa K, Lin SF, Tojo A. Tsai HJ, et al. Cancer Sci. 2011 Nov;102(11):2014-21. doi: 10.1111/j.1349-7006.2011.02039.x. Epub 2011 Sep 1. Cancer Sci. 2011. PMID: 21777350 Free PMC article. - Pim1 serine/threonine kinase regulates the number and functions of murine hematopoietic stem cells.
An N, Lin YW, Mahajan S, Kellner JN, Wang Y, Li Z, Kraft AS, Kang Y. An N, et al. Stem Cells. 2013 Jun;31(6):1202-12. doi: 10.1002/stem.1369. Stem Cells. 2013. PMID: 23495171 Free PMC article. - Survival prognostic factors in patients with acute myeloid leukemia using machine learning techniques.
Karami K, Akbari M, Moradi MT, Soleymani B, Fallahi H. Karami K, et al. PLoS One. 2021 Jul 21;16(7):e0254976. doi: 10.1371/journal.pone.0254976. eCollection 2021. PLoS One. 2021. PMID: 34288963 Free PMC article. - GATA2 regulates differentiation of bone marrow-derived mesenchymal stem cells.
Kamata M, Okitsu Y, Fujiwara T, Kanehira M, Nakajima S, Takahashi T, Inoue A, Fukuhara N, Onishi Y, Ishizawa K, Shimizu R, Yamamoto M, Harigae H. Kamata M, et al. Haematologica. 2014 Nov;99(11):1686-96. doi: 10.3324/haematol.2014.105692. Epub 2014 Aug 22. Haematologica. 2014. PMID: 25150255 Free PMC article. - Differentiation therapy of leukemia: 3 decades of development.
Nowak D, Stewart D, Koeffler HP. Nowak D, et al. Blood. 2009 Apr 16;113(16):3655-65. doi: 10.1182/blood-2009-01-198911. Epub 2009 Feb 12. Blood. 2009. PMID: 19221035 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous