High LET radiation enhances apoptosis in mutated p53 cancer cells through Caspase-9 activation - PubMed (original) (raw)
High LET radiation enhances apoptosis in mutated p53 cancer cells through Caspase-9 activation
Nobuhiro Yamakawa et al. Cancer Sci. 2008 Jul.
Abstract
Although mutations in the p53 gene can lead to resistance to radiotherapy, chemotherapy and thermotherapy, high linear energy transfer (LET) radiation induces apoptosis regardless of p53 gene status in cancer cells. The aim of this study was to clarify the mechanisms involved in high LET radiation-induced apoptosis. Human gingival cancer cells (Ca9-22 cells) containing a mutated p53 (mp53) gene were irradiated with X-rays, C-ion (13-100 KeV/microm), or Fe-ion beams (200 KeV/microm). Cellular sensitivities were determined using colony forming assays. Apoptosis was detected and quantified with Hoechst 33342 staining. The activity of Caspase-3 was analyzed with Western blotting and flow cytometry. Cells irradiated with high LET radiation showed a high sensitivity with a high frequency of apoptosis induction. The relative biological effectiveness (RBE) values for the surviving fraction and apoptosis induction increased in a LET-dependent manner. Both RBE curves reached a peak at 100 KeV/microm, and then decreased at values over 100 KeV/microm. When cells were irradiated with high LET radiation, Caspase-3 was cleaved and activated, leading to poly (ADP-ribose) polymerase (PARP) cleavage. In addition, Caspase-9 inhibitor suppressed Caspase-3 activation and apoptosis induction resulting from high LET radiation to a greater extent than Caspase-8 inhibitor. These results suggest that high LET radiation enhances apoptosis by activation of Caspase-3 through Caspase-9, even in the presence of mp53.
Figures
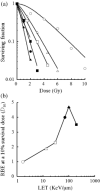
Figure 1
Surviving fraction after exposure to X‐rays or to heavy‐ion beams. (a) Radiation sensitivity; (b) RBE at D 10. Cells were irradiated with X‐rays ( ) or C‐ion beams with LET values of 13 KeV/µm (Δ), 30 KeV/µm (
) or C‐ion beams with LET values of 13 KeV/µm (Δ), 30 KeV/µm ( ), 70 KeV/µm (
), 70 KeV/µm ( ), and 100 KeV/µm (
), and 100 KeV/µm ( ), and Fe‐ion beams with LET values of 200 KeV/µm (
), and Fe‐ion beams with LET values of 200 KeV/µm ( ). The data are presented as the means and standard errors of three independent experiments.
). The data are presented as the means and standard errors of three independent experiments.
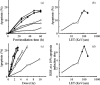
Figure 2
Time‐ and dose‐dependent apoptosis induction after exposure to X‐rays or heavy‐ion beams. (a) Time course of apoptosis induced by X‐ray and heavy‐ion beam irradiation with 2 Gy. (b) The induction of apoptosis was investigated after irradiation with beams of various LET values at 48 h after a 2 Gy irradiation. (c) The induction of apoptosis as a function of dose. The data are presented as the means and standard errors of three independent experiments. (d) Relative biological effectiveness at a dose which results in 10% apoptosis induction. Cells were irradiated with X‐rays ( ), C‐ion beams with LET values of 13 KeV/µm (Δ), 30 KeV/µm (
), C‐ion beams with LET values of 13 KeV/µm (Δ), 30 KeV/µm ( ), 70 KeV/µm (
), 70 KeV/µm ( ), 100 KeV/µm (
), 100 KeV/µm ( ), and Fe‐ion beams with LET value of 200 KeV/µm (
), and Fe‐ion beams with LET value of 200 KeV/µm ( ). The data are presented as the means and standard errors of three independent experiments.
). The data are presented as the means and standard errors of three independent experiments.
Figure 3
Caspase‐3 activity after exposure to X‐rays or heavy‐ion beams. Fragmentation of PARP and Caspase‐3 at 48 h after a 2 Gy irradiation is shown.
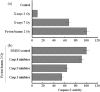
Figure 4
Caspase‐3 activity after exposure to X‐rays or Fe‐beams in viable cells. Caspase‐3 activity was analyzed at 48 h after irradiation. (a) without Caspase inhibitors, and (b) with Caspase‐3, Caspase‐8, and, Caspase‐9 inhibitors (at 20 µM concentrations). The data are presented as the means and standard errors of three independent experiments.
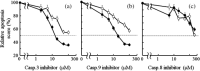
Figure 5
Effect of Caspase inhibitors on apoptosis induction. Induction of apoptosis was investigated at 48 h after D 10 irradiation in the presence of inhibitors for (a) Caspase‐3 (2–100 µM) (b) Caspase‐8 (5–200 µM), and (c) Caspase‐9 (2–100 µM). Cells were irradiated with C‐ion beams with LET values of 100 KeV/µm ( ) and X‐rays (
) and X‐rays ( ). Relative apoptosis values were calculated as apoptosis induction with or without inhibitors. The data are presented as the means and standard errors of three independent experiments.
). Relative apoptosis values were calculated as apoptosis induction with or without inhibitors. The data are presented as the means and standard errors of three independent experiments.
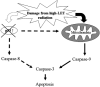
Figure 6
A model for high LET radiation‐induced _p53_‐independent apoptosis. Apoptotic pathways triggered by high LET radiation do not require p53. Any damage induced by high LET radiation is proposed to be a critical trigger for the activation of the Caspase‐9‐related apoptotic pathway, rather than the Caspase‐8 related pathway. Caspase‐3 is activated by Caspase‐9 leading to _p53_‐independent apoptosis. In addition, Caspase‐8 is not activated because p53 is defective and does not respond in a normal manner.
Similar articles
- Depression of p53-independent Akt survival signals in human oral cancer cells bearing mutated p53 gene after exposure to high-LET radiation.
Nakagawa Y, Takahashi A, Kajihara A, Yamakawa N, Imai Y, Ota I, Okamoto N, Mori E, Noda T, Furusawa Y, Kirita T, Ohnishi T. Nakagawa Y, et al. Biochem Biophys Res Commun. 2012 Jul 13;423(4):654-60. doi: 10.1016/j.bbrc.2012.06.004. Epub 2012 Jun 10. Biochem Biophys Res Commun. 2012. PMID: 22695120 - p53-dependent thermal enhancement of cellular sensitivity in human squamous cell carcinomas in relation to LET.
Takahashi A, Ohnishi K, Ota I, Asakawa I, Tamamoto T, Furusawa Y, Matsumoto H, Ohnishi T. Takahashi A, et al. Int J Radiat Biol. 2001 Oct;77(10):1043-51. doi: 10.1080/09553000110066095. Int J Radiat Biol. 2001. PMID: 11682009 - Apoptosis induced by high-LET radiations is not affected by cellular p53 gene status.
Takahashi A, Matsumoto H, Furusawa Y, Ohnishi K, Ishioka N, Ohnishi T. Takahashi A, et al. Int J Radiat Biol. 2005 Aug;81(8):581-6. doi: 10.1080/09553000500280484. Int J Radiat Biol. 2005. PMID: 16298939 - [Analysis of death pattern in cancer cells by using different kinds of LET radiation].
Takahashi A, Ohnishi K, Aoki M, Furusawa Y, Ohnishi T. Takahashi A, et al. Nihon Igaku Hoshasen Gakkai Zasshi. 2002 Sep;62(10):531-4. Nihon Igaku Hoshasen Gakkai Zasshi. 2002. PMID: 12391680 Review. Japanese. - High LET heavy ion radiation induces p53-independent apoptosis.
Mori E, Takahashi A, Yamakawa N, Kirita T, Ohnishi T. Mori E, et al. J Radiat Res. 2009 Jan;50(1):37-42. doi: 10.1269/jrr.08075. Epub 2008 Oct 29. J Radiat Res. 2009. PMID: 18957831 Review.
Cited by
- Carbon ions of different linear energy transfer (LET) values induce apoptosis & G2 cell cycle arrest in radio-resistant melanoma cells.
Jelena Ž, Lela K, Otilija K, Danijela T, Cirrone Giuseppe AP, Francesco R, Giacomo C, Ivan P, Aleksandra RF. Jelena Ž, et al. Indian J Med Res. 2016 May;143(Supplement):S120-S128. doi: 10.4103/0971-5916.191811. Indian J Med Res. 2016. PMID: 27748286 Free PMC article. - Effect of silencing of high mobility group A2 gene on gastric cancer MKN-45 cells.
Wei CH, Wei LX, Lai MY, Chen JZ, Mo XJ. Wei CH, et al. World J Gastroenterol. 2013 Feb 28;19(8):1239-46. doi: 10.3748/wjg.v19.i8.1239. World J Gastroenterol. 2013. PMID: 23482887 Free PMC article. - MEK-ERK-dependent multiple caspase activation by mitochondrial proapoptotic Bcl-2 family proteins is essential for heavy ion irradiation-induced glioma cell death.
Tomiyama A, Tachibana K, Suzuki K, Seino S, Sunayama J, Matsuda KI, Sato A, Matsumoto Y, Nomiya T, Nemoto K, Yamashita H, Kayama T, Ando K, Kitanaka C. Tomiyama A, et al. Cell Death Dis. 2010 Jul 29;1(7):e60. doi: 10.1038/cddis.2010.37. Cell Death Dis. 2010. PMID: 21364665 Free PMC article. - High-LET charged particles: radiobiology and application for new approaches in radiotherapy.
Helm A, Fournier C. Helm A, et al. Strahlenther Onkol. 2023 Dec;199(12):1225-1241. doi: 10.1007/s00066-023-02158-7. Epub 2023 Oct 23. Strahlenther Onkol. 2023. PMID: 37872399 Free PMC article. Review. - Therapeutic strategies for head and neck cancer based on p53 status.
Ota I, Okamoto N, Yane K, Takahashi A, Masui T, Hosoi H, Ohnishi T. Ota I, et al. Exp Ther Med. 2012 Apr;3(4):585-591. doi: 10.3892/etm.2012.474. Epub 2012 Feb 3. Exp Ther Med. 2012. PMID: 22969933 Free PMC article.
References
- Palme CE, Gullane PJ, Gilbert RW. Current treatment options in squamous cell carcinoma of the oral cavity. Surg Oncol Clin North Am 2004; 13: 47–70. - PubMed
- Cooper JS, Pajak TF, Forastiere AA et al . Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med 2004; 350: 1937–44. - PubMed
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253: 49–53. - PubMed
- Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle 2007; 6: 1006–10. - PubMed
- Vousden KH, Lu X. Live or let die: the cell's response to p53 . Nat Rev Cancer 2002; 2: 594–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous