A pathway for phagosome maturation during engulfment of apoptotic cells - PubMed (original) (raw)
A pathway for phagosome maturation during engulfment of apoptotic cells
Jason M Kinchen et al. Nat Cell Biol. 2008 May.
Abstract
Removal of apoptotic cells is critical for the physiological well-being of the organism and defects in corpse removal have been linked to disease states. Genes regulating corpse recognition and internalization have been identified, but few molecules involved in the processing of internalized corpses are known. Through a combination of targeted and unbiased reverse genetic screens in Caenorhabditis elegans, and studies in mammalian cells, we have identified genes required for maturation of apoptotic-cell-containing phagosomes. We have further ordered these candidates, which include the GTPases RAB-5 and RAB-7 and the HOPS complex, into a coherent linear pathway for the maturation of apoptotic cells within phagosomes. In depth analysis of two additional candidate genes, the phosphatidylinositol 3 kinase (PI(3)K) vps-34 (A001762) and dyn-1/dynamin, showed an accumulation of internalized, but undegraded, corpses within abnormal Rab5-negative phagosomes. We ordered these candidates in our pathway, with DYN-1 functioning upstream of VPS-34 in the recruitment and/or retention of RAB-5 to the phagosome. Finally, we have also identified a previously undescribed biochemical complex containing Vps34, dynamin and Rab5(GDP), thus providing a mechanism for Rab5 recruitment to the nascent phagosome.
Figures
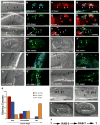
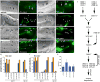
Figure 1. RAB-5 and RAB-7 are required for efficient corpse clearance
Bright field images represent DIC micrographs. Arrows and arrowheads indicate apoptotic germ cells or the indicated fluorescently tagged protein localized around apoptotic germ cells in dissected gonads. Chart shows number of halos normalized to corpse number; Error bars represent s.e.m. n>10 animals for each condition. SYTO41 or SYTO59 dye was used according to spectral requirements for co-localization with YFP or CFP. Size bar, 10 Îm. (aâl) In wild-type worms, CFP::RAB-5 (aâd) and YFP::RAB-7 (eâh) highlight SYTO-positive, late stage internalized germ cell corpses (d, h, arrowheads) as well as corpses that stain weakly with SYTO dyes (c, g). Occasionally, more than one corpse can be seen as RAB-5 or RAB-7 positive structures (d, h, asterisks). YFP::RAB-5 (j, arrows) and CFP::RAB-7 (k, arrowheads) stain distinct sets of phagosomes (l), though the two makers are occasionally seen on the same phagosome (l, asterisk). (mân) 1.5-fold embryos were assessed for localization of YFP::RAB-5 (m, mâ, arrowheads) and YFP::RAB-7 (n, nâ, arrowheads) around phagosomes containing apoptotic cells arising during developmental morphogenesis. (oâr) Mutation of either ced-1 or ced-5 severely reduces recruitment of CFP::RAB-5 (oâ, pâ) and YFP::RAB-7 (qâ, râ) around apoptotic cell corpses (oâr, arrowheads). (s) RAB-5 and RAB-7 stain discrete stages during corpse engulfment, with RAB-5 preferentially localizing around early corpses and RAB-7 localizing around late apoptotic cell corpses (quantitated in s). Corpse staging is described in Supplementary Figure S2. (tâw) RNA interference against either rab-5 (t) or rab-7 (u), but not control RNAi (v and data not shown) resulted in increased numbers of undegraded refractile corpses in the adult hermaphrodite gonad or during developmental morphogenesis (w). Note: rab-5(RNAi) resulted in incompletely penetrant larval arrest; the animals scored (t) represent escapers that grew into adults. Corpse number is quantitated in Table 1. (x) Schematic of the pathway for phagosome maturation containing apoptotic cells, based on the data above.
Figure 2. The HOPS complex functions downstream of RAB-7 during maturation of phagosomes containing apoptotic cells
Bright field images represent DIC micrographs. Arrows and arrowheads indicate apoptotic germ cells or protein localized around apoptotic germ cells; asterisks represent RAB-7 staining phagosomes that contain non-refractile, partially degraded apoptotic cells. Error bars represent s.d. Size bar represents 10 Îm. (aâf) RNA interference against indicated members of the HOPS complex resulted in increased numbers of undegraded, refractile cell corpses in the gonad. (g) The increase in number of YFP::RAB-5 and YFP::RAB-7 positive phagosomes (as a marker for arrest in maturation of phagosomes containing corpses) was quantitated in animals treated with RNAi targeting ced-1, rab-7 or indicated members of the HOPS complex In rab-7(RNAi) animals, there is an accumulation of corpses in RAB-5(+) phagosomes, while the failure to see halos in ced-1 RNAi is due to defects in early stages of engulfment. (hâs) The number of RAB-5-positive phagosomes in animals treated with vps-11 and vps-39 RNAi (m,q) were similar to control RNAi treated nematodes (i), while the number of RAB-7-positive phagosomes (o, s) were increased compared to control (k). Quantitated in (g). (t) Schematic of the genetic pathway for phagosome maturation containing apoptotic cells, based on the data above.
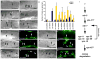
Figure 3. A reverse genetic screen identifies DYN-1, which is required for efficient corpse removal and localizes around apoptotic cells in the adult hermaphrodite gonad
Bright field images represent DIC micrographs. Arrows/arrowheads indicate refractile cell corpses in the DIC images or the indicated protein for localization around apoptotic cells in the fluorescence images. dyn-1(ky51) mutant worms are shown at the nonpermissive temperature. Scale bar, 10 _Î_m. (aâd) In wild-type worms (a, b), acridine orange (AO) preferentially stains engulfed apoptotic cells present in acidic compartments. In worms mutant for genes required for efficient removal of apoptotic cells, refractile cell corpses persist but do not stain with AO [ced-1(e1735), c, d]. (e) Schematic of reverse genetic screen. Worms were fed bacteria expressing dsRNA as at the L1 larval stage, then stained with AO as adults. AO negative candidates were then compared to previous RNAi screens to exclude false negatives due to sterility or other gonadal defects. Genes identified were assigned to categories based on proposed function (e.g., cell architecture). Finally, refractile cell corpses were scored in the 12-hour adult hermaphrodite gonad by DIC microscopy (Supplementary Table S1). (fâk) Inactivation of candidate genes identified in the screen, e.g. dyn-1(ky51) (f, g) and vps-34(RNAi) (h, i) showed persistent corpses without AO staining (g, i, arrowheads). rab-7(ok511) showed corpses arrested in AO-staining phagosomes (j, k). (lâu) DYN-1 is recruited around the apoptotic cell during phagocytosis (l, m, arrowhead), but not when the engulfment process is disrupted, as in ced-1 (n, o) and ced-5 (p, q) deficient worms. In vps-34 (r, s) and rme-8 (t, u) deficient worms, DYN-1 is still recruited, suggesting DYN-1 may function upstream of VPS-34 and RME-8 during corpse removal. Quantitation of the data presented here is shown in Supplementary Table S2. (v) DYN-1 is recruited to the phagocytic cup and associated with early phagosomes. DYN-1::CFP colocalizes with YFP::actin around early/intermediate stage apoptotic cell corpses (v and the appropriate subpanels). (wâz) DYN-1 is recruited around SYTO 41-negative (early) apoptotic cell corpses (w, x, z, overlay, inset, arrow) and is not present on late, SYTO41-positive corpses (y, overlay, inset, arrowhead). SYTO 41, like acridine orange, preferentially stains late-stage, internalized apoptotic cells.
Figure 4. Apoptotic cells are efficiently internalized in dyn-1 mutant worms
Bright field images represent DIC micrographs. dyn-1(ky51) mutant worms are scored at the nonpermissive temperature (25ÂC) unless otherwise stated. Arrows, arrowheads indicate apoptotic cells. Scale bar, 10 Î_m. Error bars represent s.d. (aâj) Normal recruitment of CED-1 and actin polymerization around corpses in dyn-1(ky51) mutant worms at 25ÂC. Both CED-1::GFP recruitment, which marks the phagocytic cup (b, inset), and actin reorganization (e, arrows) during engulfment appear normal in dyn-1(ky51) worms at the non-permissive temperature (d, h, arrows), while ced-1(e1735) mutant worms display a severe defect in actin polymerization and internalization of the apoptotic cell (j, arrows). Residual staining in the sheath cells represents cortical actin filaments or G-actin. Quantitation of this data is presented in (q). (kâp) In wild type worms, actin stress fibers tend to run longitudinally in the proximal gonad (k and camera lucida, l). However, after long-term knockdown with dyn-1(RNAi) (48 hours), animals show distorted, disoriented actin fibers (m, n), which may have resulted in the phagocytic detected at these time points (Supplementary Figure S5). In dyn-1(ky51) mutant worms,_ incubated for 24 hours at 25ÂC, the actin fiber pattern appears as wild type (o,p).
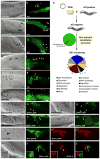
Figure 5. Dynamin in phagocytes is recruited around apoptotic cells coincident with corpse internalization
CFSE/TAMRA stains the cytoplasm of apoptotic cells, frequently resulting in a lunette of staining around the nucleus. Arrows and asterisks indicate apoptotic cells. Scale bar, 10 Îm. Error bars represent s.d. (aâh) J774 macrophages (aâd) or NIH/3T3 fibroblasts (eâh) were incubated with apoptotic thymocytes or apoptotic Jurkat cells, respectively, and localization of endogenous dynamin (green) and polymerized actin (red) were monitored. Dynamin was recruited to the phagocytic cup with actin in J774 (15 min) (d, merge) and NIH/3T3 cells (30 min) (h, merge) (indicated by arrow), but was not recruited around bound apoptotic cells (d, arrowhead). (iân) Confocal z-sections were reconstructed to generate yz planes (m, n). J774 macrophages incubated with apoptotic cells at 4 ÂC did not show phagocytic cup formation or enrichment of endogenous dynamin around the apoptotic cell (Supplementary Figure S6). Cells incubated at 37 ÂC showed dynamin localized in the phagocytic cup (m, arrowhead and n, camera lucida) adjacent to the apoptotic cell in a punctate pattern (j, k, inset). Dotted lines (l) indicate plane of yz reconstruction. (o) Time course of endogenous dynamin recruitment around the apoptotic cell corpse in J774 macrophages. Cells that had bound apoptotic cells were scored positive for dynamin recruitment if a âhaloâ of dynamin was seen to surround the apoptotic cell. 4 ÂC, n=100 (4 experiments); 37 ÂC, n=200 (8 experiments).
Figure 6. Dynamin is required for maturation of engulfed apoptotic cells into Rab5-coated endosomes
Arrows and arrowheads indicate apoptotic cells or protein recruited around engulfed apoptotic cells in phagosomes. Neither Dyn2K44A nor Dyn2RNAi had any obvious effect on staining of endogenous endosomal/lysosomal structures with Lysotracker Red or Rab5. Error bars represent s.d. Scale bar, 10 _Î_m. (aâl) NIH/3T3 fibroblasts transfected with GFP (aâd), HA-Dyn2K44A (eâh), or GFP-Rab5S34N (iâl) were incubated with apoptotic thymocytes in the presence of Lysotracker Red to determine the efficiency of phagosome maturation. In the majority of GFP-transfected cells, internalized apoptotic thymocytes (arrows) co-stained with Lysotracker red (d, inset); cells transfected with Dyn2K44A (h, inset) or Rab5S34N (l, inset) showed decreased numbers of engulfed thymocytes co-staining with Lysotracker. Apoptotic cells incubated at 4 ÂC with phagocytes did not stain strongly with Lysotracker Red (u, Supplementary Figure S9). HA-Dyn2K44A expressing cells were stained with anti-HA and an Alexa 488-conjugated secondary antibody for visualization of transfected cells. (mât) Apoptotic cells were incubated with NIH/3T3 fibroblasts, transfected with either GFP alone (as control) or HA-Dyn2K44A and the localization of endogenous Rab5 was monitored. The majority of engulfed apoptotic thymocytes inside GFP-transfected cells were in endosomes coated with Rab5 (o, arrowheads**, p**, inset). In Dyn2K44A transfected cells, phagosome maturation was disrupted and decreased numbers of engulfed thymocytes were in Rab5 coated endosomes (s, t, arrowhead, inset). Apoptotic cells incubated at 4 ÂC with phagocytes did not stain strongly for Rab5 (w, Supplementary Figure S9) (u) Quantitation of experiment shown in (aâl). 4 ÂC _n_=20 (2 experiments), 37 ÂC, n=60 (3 experiments). (v) NIH/3T3 fibroblasts were electroporated with a control siRNA or dynamin-2 (dyn2) siRNA and assayed as in (aâl). Dyn2siRNA cells showed decreased co-localization with Lysotracker as compared to controlsiRNA cells. n=50 (2 experiments). (w) Quantitation of experiment shown in (mât). 4 ÂC _n_=20 (2 experiments), 37 ÂC, n=60 (4 experiments).
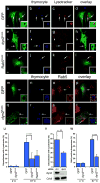
Figure 7. DYN-1 is required for efficient recruitment of RAB-5 and RAB-7 to phagosomes containing engulfed apoptotic cells in the C. elegans gonad
Bright field images represent DIC micrographs. Arrows and arrowheads indicate apoptotic germ cells or protein localized around apoptotic germ cells. dyn-1(ky51) worms are shown at the nonpermissive temperature (25 ÂC) unless otherwise stated. Scale bar, 10 _Î_m. n >10 for each transgenic. Error bars represent s.e.m. Compared to wild type (aâd), CFP::RAB-5 (e,f) and YFP::RAB-7 (g,h) halos are decreased in the gonad of dyn-1(ky51) mutant worms (f,h, arrowheads); quantitated data is presented in (q). Further, phagosomes appear larger in dyn-1(ky51) mutant worms (e, g, arrowhead and volume quantitated in (s). Nematodes deficient in vps-34 (iâl) or rme-8 (mâp) showed similar decreases in the number of RAB-5 or RAB-7 corpses (quantitated in r). (t) Schematic of the genetic pathway for phagosome maturation containing apoptotic cells, based on the data presented above and in earlier figures.
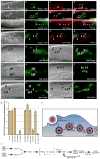
Figure 8. Vps34 functions downstream of Dyn2 and mediates the interaction between Dyn2 and Rab5
Arrows indicate localization of phagocytic cup or co-localizaiton of indicated proteins. Size bar, 10 _Î_m. Apoptotic cells were stained with CMHC, whose fluorescence becomes less detectable when the apoptotic cells are engulfed (g, arrowhead). (aâd) Apoptotic Jurkat cells were incubated with NIH/3T3 fibroblasts transiently transfected with FLAG-Vps34 and the localization of endogenous Dyn2 (a) and FLAG-Vps34 (b) in phagocytic cups containing apoptotic cells (c) were monitored. Both proteins co-localize in the phagocytic cup (d, overlay, arrow) (eân) Apoptotic Jurkat cells were incubated with NIH/3T3 fibroblasts transfected with either GFP alone (as control, not shown) or HA-tagged Dyn2WT (e) or Dyn2K44A (j) and the localization of FLAG-tagged Vps34 (f, k) on phagocytic cups containing apoptotic cells (g, l) was monitored. In Dyn2K44A transfected cells, recruitment of FLAG-Vps34 to the forming phagosome was disrupted (k, arrow), while Vps34 was efficiently recruited to the phagocytic cup in Dyn2WT (f) or GFP-transfected cells (not shown). Phalloidin-stained cells are included to better discriminate phagocytic cups (i, n), which are enriched in actin. Quantitation of these data are presented in (o). (p) 293T cells were transiently transfected with the indicated proteins, lysed and immunoprecipitated using anti-Flag conjugated agarose beads and assessed by immunoblotting for the indicated proteins. (q) GST-tagged Vps34 or ELMO1 proteins were expressed in bacteria and purified using GST-sepharose. Approximately 10 _Î_g purified His-Dyn2 was added to approximately 2 _Î_g of each GST-tagged protein. Protein interaction was assessed by immunoblotting. (r) 293T cells were transiently transfected with the indicated proteins, lysed and immunoprecipitated using anti-GFP conjugated agarose beads and assessed by immunoblotting for the coprecipitating proteins. Rab5Q79L and Rab5S34N are considered to mimic for GTP and GDP-bound Rab5, respectively.
Figure 9. DYN-1 co-localizes with RAB-5 and is required for VPS-34 activity
Bright field images represent DIC micrographs. Arrows and arrowheads indicate apoptotic germ cells or protein localized around apoptotic germ cells. dyn-1(ky51) worms are shown at the nonpermissive temperature (25 ÂC) unless otherwise stated. Scale bar, 10 _Î_m. n >10 for each transgenic. Error bars represent s.e.m. (aâp) DYN-1::YFP (b, arrow) and CFP::RAB-5 (c, arrow) co-localize on phagosomes containing apoptotic cells (a, arrow and d, overlay) at similar times. In rab-7(RNAi) worms, phagosome-containing corpses (e) are arrested at the RAB-5(+) stage (g, arrows and arrowheads); a population of these stain with DYN-1::YFP (f, h, arrows), suggesting proteins transiently stain the same phagosome as it matures. vps-34(RNAi) inhibits co-localization of DYN-1::YFP (j, l, arrowheads) and CFP::RAB-5 (k, l, arrowheads). DYN-1::YFP and CFP::RAB-7 did not co-localize on apoptotic cell containing phagosomes (mâp, arrowheads). (qâv) The YFP::2xFYVE construct specifically binds domains enriched in PtdIns(3)P. YFP::2xFYVE is recruited to phagosomes containing early apoptotic corpses (q, pre-refractile corpse, arrow), while it is excluded from phagosomes containing late corpses (refractile, arrow head). 2xFYVE::YFP is recruited to phagosomes (q, qâ, arrows) in wild type worms; this recruitment is greatly decreased in ced-6(n1813) (r, râ), dyn-1(ky51) (s, sâ), and rab-5(RNAi) (u, uâ) worms. Recruitment to phagosomes is greatly decreased in vps-34(RNAi) worms (t, tâ), consistent with a role for VPS-34 in generation of PtdIns(3)P. 2xFYVE::YFP is still recruited in rab-7(RNAi) (v, vâ). These data were quantitated in (w). (x, y) Potential model for maturation of phagosomes containing apoptotic cells based on cell biological (x) and genetic studies (y) in C. elegans and mammals presented here.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - "I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.
Huang Y, Trollor JN, Foley KR, Arnold SRC. Huang Y, et al. Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - "It Is a Big Spider Web of Things": Sensory Experiences of Autistic Adults in Public Spaces.
MacLennan K, Woolley C, Andsensory E, Heasman B, Starns J, George B, Manning C. MacLennan K, et al. Autism Adulthood. 2023 Dec 1;5(4):411-422. doi: 10.1089/aut.2022.0024. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116051 Free PMC article. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- Programmed cell death and clearance of cell corpses in Caenorhabditis elegans.
Wang X, Yang C. Wang X, et al. Cell Mol Life Sci. 2016 Jun;73(11-12):2221-36. doi: 10.1007/s00018-016-2196-z. Epub 2016 Apr 5. Cell Mol Life Sci. 2016. PMID: 27048817 Free PMC article. Review. - Involvement of Beclin 1 in engulfment of apoptotic cells.
Konishi A, Arakawa S, Yue Z, Shimizu S. Konishi A, et al. J Biol Chem. 2012 Apr 20;287(17):13919-29. doi: 10.1074/jbc.M112.348375. Epub 2012 Mar 5. J Biol Chem. 2012. PMID: 22393062 Free PMC article. - Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages.
Miettinen M, Pietilä TE, Kekkonen RA, Kankainen M, Latvala S, Pirhonen J, Österlund P, Korpela R, Julkunen I. Miettinen M, et al. Gut Microbes. 2012 Nov-Dec;3(6):510-22. doi: 10.4161/gmic.21736. Epub 2012 Aug 16. Gut Microbes. 2012. PMID: 22895087 Free PMC article. - Mesenchymal stem cell-derived apoptotic bodies alleviate alveolar bone destruction by regulating osteoclast differentiation and function.
Li X, Jiang Y, Liu X, Fu J, Du J, Luo Z, Xu J, Bhawal UK, Liu Y, Guo L. Li X, et al. Int J Oral Sci. 2023 Dec 1;15(1):51. doi: 10.1038/s41368-023-00255-y. Int J Oral Sci. 2023. PMID: 38040672 Free PMC article. - Role of transcription factors in apoptotic cells clearance.
Gao Y, Jiao Y, Gong X, Liu J, Xiao H, Zheng Q. Gao Y, et al. Front Cell Dev Biol. 2023 Jan 19;11:1110225. doi: 10.3389/fcell.2023.1110225. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36743409 Free PMC article. Review.
References
- Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. - PubMed
- Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. - PubMed
- Hanayama R, Miyasaka K, Nakaya M, Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr Dir Autoimmun. 2006;9:162–72. - PubMed
- Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. - PubMed
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM064709/GM/NIGMS NIH HHS/United States
- R01 GM064709-07/GM/NIGMS NIH HHS/United States
- R01 GM069998/GM/NIGMS NIH HHS/United States
- R01 GM069998-04/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous