Proliferating mesodermal cells in murine embryos exhibiting macrophage and lymphendothelial characteristics - PubMed (original) (raw)
Proliferating mesodermal cells in murine embryos exhibiting macrophage and lymphendothelial characteristics
Kerstin Buttler et al. BMC Dev Biol. 2008.
Abstract
Background: The data on the embryonic origin of lymphatic endothelial cells (LECs) from either deep embryonic veins or mesenchymal (or circulating) lymphangioblasts presently available remain inconsistent. In various vertebrates, markers for LECs are first expressed in specific segments of embryonic veins arguing for a venous origin of lymph vessels. Very recently, studies on the mouse have strongly supported this view. However, in the chick, we have observed a dual origin of LECs from veins and from mesodermal lymphangioblasts. Additionally, in murine embryos we have detected mesenchymal cells that co-express LEC markers and the pan-leukocyte marker CD45. Here, we have characterized the mesoderm of murine embryos with LEC markers Prox1, Lyve-1 and LA102 in combination with macrophage markers CD11b and F4/80.
Results: We observed cells co-expressing both types of markers (e.g. Prox1 - Lyve-1 - F4/80 triple-positive) located in the mesoderm, immediately adjacent to, and within lymph vessels. Our proliferation studies with Ki-67 antibodies showed high proliferative capacities of both the Lyve-1-positive LECs of lymph sacs/lymphatic sprouts and the Lyve-1-positive mesenchymal cells.
Conclusion: Our data argue for a dual origin of LECs in the mouse, although the primary source of embryonic LECs may reside in specific embryonic veins and mesenchymal lymphangioblasts integrated secondarily into lymph vessels. The impact of a dual source of LECs for ontogenetic, phylogenetic and pathological lymphangiogenesis is discussed.
Figures
Figure 1
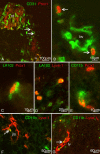
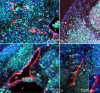
Jugular lymph sacs of murine embryos. Lyve-1 staining of paraffin sections (A, B) and double staining of cryo-sections (C-F) of ED 13.5 (A-C, E, F) and 12.5 (D) mice. A, B) The jugular lymph sacs (jls) are Lyve-1+ and located dorso-laterally of the cardinal vein (v). Many scattered Lyve-1+ cells are located superficially in the developing dermis (arrows). nt, neural tube. B) Higher magnification of A showing JLS in close proximity to the cardinal vein. C) Lymph sacs express Prox1 (red) and Lyve-1 (green). D) Prox1 (red) and LA102 (green). E) Lyve-1 (red) and LA102 (green). Note double positive LECs, although staining of lymph sac LECs with LA102 is heterogeneous. F) Lymphatic vessel (arrow) in the dermis is double positive for Lyve-1 and LA102 (yellow).
Figure 2
Double staining of scattered mesodermal cells with pan-endothelial, lymphendothelial and macrophage markers of ED 10 (A, B), ED 12.5 (D, E) and ED 13.5 (C, F, G) mouse embryos. A) Two mesenchymal cells of the dermatome (arrows) which are located at a great distance from the cardinal vein (v) express CD31 (green) and Prox1 (red). nt, neural tube. B) Higher magnification of A). Note weak CD31 expression of Prox1+ mesenchymal cells (arrows) as compared to blood vessels (bv). C) Cells double positive for lymphendothelial markers LA102 (green) and Prox1 (red), and D) LA102 (green) and Lyve-1 (red). E) Cells double positive for macrophage marker CD11b (green) and lymphendothelial marker Prox1 (red). F) Some cells co-express (yellow) CD11b (green) and Lyve-1 (red) in the mesenchyme adjacent to the JLS, and G) in the dermis, where a cell positive for both markers (arrow) seems to be integrated into a lymph vessel (lv).
Figure 3
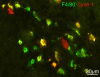
F4/80 (green) and Lyve-1 (red) staining of ED 13.5 mice. Many of the cells in the dermis are double positive (yellow), but some only express F4/80 or Lyve-1.
Figure 4
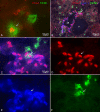
Double and triple staining of ED 11.5 (A) and ED 13.5 (B – F) mouse embryos with Prox1 (red), Lyve-1 (blue), and F4/80 (green). A) Cell showing co-expression of Prox1 and F4/80 (arrow), whereas another cell is only F4/80 positive. B) Overview showing the jugular lymph sac (jls) and the cardinal vein (v). Prox1 and Lyve-1 are co-expressed in LECs of the jls. Arrow indicates integration of F4/80+ cell into the jls. C) Higher magnification of B) showing triple positive cells of the JLS. D) Prox1, E) Lyve-1, F) F4/80.
Figure 5
Triple staining of ED 13.5 mouse embryos with Prox1 (red), Lyve-1 (blue), and F4/80 (green). A, B) Triple positive cell integrated in a lymphatic vessel (arrow in A and B). A) Cell showing co-expression of Lyve-1 and F4/80 (white arrowhead), whereas another cell is only F4/80 positive (open arrowhead).
Figure 6
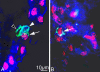
Paraffin sections of mouse embryos stained with antibodies against Ki-67 (green) and Lyve-1 (red). Counter staining was performed with DAPI (blue). A) On ED 11.5, there are proliferating LECs in the developing jugular lymph sacs (jls) (arrows), but no double positive cells in the cardinal vein (v). B) Two mesenchymal cells positive for the markers located in the dermatome (arrows). g, spinal ganglion. C) JLS of ED 12.5 mice with LECs (arrows) expressing Ki-67 and Lyve-1. D) Sprout from the JLS containing proliferating cells (arrows). Inset: Higher magnification showing Ki-67-positive (arrow) and negative (arrowhead) LECs.
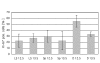
Figure 7
Proliferation studies of Lyve-1+ cells with Ki-67 antibodies in ED 12.5 and ED 13.5 mice. For each ED, at least 5 sections of at least 3 mice were evaluated. The total number of cells counted was: 197 in lymph sacs (LS) on ED 12.5; 406 in lymph sacs (LS) on ED 13.5; 45 in sprouts from lymph sacs (Sp) on ED 12.5; 75 in sprouts from lymph sacs (Sp) on ED 13.5; 43 in scattered cells in the dermatomes (D) on ED 12.5 and 40 in scattered cells in the dermatomes (D) on ED13.5. Mean and standard deviation are shown. The value for D 12.5 is significantly higher than all others.
Similar articles
- Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos.
Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J. Buttler K, et al. Dev Dyn. 2006 Jun;235(6):1554-62. doi: 10.1002/dvdy.20737. Dev Dyn. 2006. PMID: 16502417 - Lymphangioblasts in embryonic lymphangiogenesis.
Wilting J, Tomarev SI, Christ B, Schweigerer L. Wilting J, et al. Lymphat Res Biol. 2003;1(1):33-40. doi: 10.1089/15396850360495673. Lymphat Res Biol. 2003. PMID: 15624319 - Embryonic development and malformation of lymphatic vessels.
Wilting J, Buttler K, Rössler J, Norgall S, Schweigerer L, Weich HA, Papoutsi M. Wilting J, et al. Novartis Found Symp. 2007;283:220-7; discussion 227-9, 238-41. doi: 10.1002/9780470319413.ch17. Novartis Found Symp. 2007. PMID: 18300425 - Myeloid cells and lymphangiogenesis.
Zumsteg A, Christofori G. Zumsteg A, et al. Cold Spring Harb Perspect Med. 2012 Jun;2(6):a006494. doi: 10.1101/cshperspect.a006494. Cold Spring Harb Perspect Med. 2012. PMID: 22675661 Free PMC article. Review. - Lymphatic endothelial cells, inflammatory lymphangiogenesis, and prospective players.
Ji RC. Ji RC. Curr Med Chem. 2007;14(22):2359-68. doi: 10.2174/092986707781745541. Curr Med Chem. 2007. PMID: 17896984 Review.
Cited by
- New model of macrophage acquisition of the lymphatic endothelial phenotype.
Hall KL, Volk-Draper LD, Flister MJ, Ran S. Hall KL, et al. PLoS One. 2012;7(3):e31794. doi: 10.1371/journal.pone.0031794. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396739 Free PMC article. - Lymphangiogenesis and angiogenesis during human fetal pancreas development.
Roost MS, van Iperen L, de Melo Bernardo A, Mummery CL, Carlotti F, de Koning EJ, Chuva de Sousa Lopes SM. Roost MS, et al. Vasc Cell. 2014 Nov 1;6:22. doi: 10.1186/2045-824X-6-22. eCollection 2014. Vasc Cell. 2014. PMID: 25785186 Free PMC article. - Host immune cellular reactions in corneal neovascularization.
Abdelfattah NS, Amgad M, Zayed AA. Abdelfattah NS, et al. Int J Ophthalmol. 2016 Apr 18;9(4):625-33. doi: 10.18240/ijo.2016.04.25. eCollection 2016. Int J Ophthalmol. 2016. PMID: 27162740 Free PMC article. Review. - Developmental and pathological lymphangiogenesis: from models to human disease.
Maby-El Hajjami H, Petrova TV. Maby-El Hajjami H, et al. Histochem Cell Biol. 2008 Dec;130(6):1063-78. doi: 10.1007/s00418-008-0525-5. Epub 2008 Oct 23. Histochem Cell Biol. 2008. PMID: 18946678 Review. - Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors.
Ran S, Montgomery KE. Ran S, et al. Cancers (Basel). 2012 Sep;4(3):618-57. doi: 10.3390/cancers4030618. Epub 2012 Jun 27. Cancers (Basel). 2012. PMID: 22946011 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous