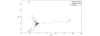Expression patterns and prognostic value of Bag-1 and Bcl-2 in breast cancer - PubMed (original) (raw)
Expression patterns and prognostic value of Bag-1 and Bcl-2 in breast cancer
Yasmine Nadler et al. Breast Cancer Res. 2008.
Abstract
Introduction: Bcl-2 antanogene-1 (Bag-1) binds the anti-apoptotic mediator Bcl-2, and enhances its activity. Bcl-2 and Bag-1 are associated with chemotherapy resistance in cancer cells. Drugs that target Bcl-2 are currently in clinical development. The purpose of the present study was to examine expression patterns of Bag-1 in a large cohort of breast tumors and to assess the association with Bcl-2, estrogen receptor, progesterone receptor and Her2/neu, and other clinical/pathological variables.
Methods: Tissue microarrays containing primary specimens from 638 patients with 10-year follow-up were employed, and the expression of Bag-1, Bcl-2, estrogen receptor, progesterone receptor and Her2/neu was assessed using our automated quantitative analysis method. We used cytokeratin to define pixels as breast cancer (tumor mask) within the array spot, and we measured biomarker expression within the mask using Cy5 conjugated antibodies.
Results: High Bcl-2 expression was associated with improved survival in the entire cohort and in the node-positive subset (P = 0.008 and P = 0.002, respectively). High Bag-1 expression was associated with improved survival in the node-positive subset (P = 0.006). On multivariable analysis, neither Bcl-2 nor Bag-1 retained their independence as prognostic markers. Strong associations were found between Bag-1, Bcl-2, estrogen receptor and progesterone receptor.
Conclusion: Bag-1 and Bcl-2 expression in breast tumors is associated with improved outcome and steroid receptor positivity. Evaluation of Bcl-2 and Bag-1 expression in breast cancer may identify a subset of patients with a favorable prognosis, who might not benefit from chemotherapy or who might benefit from Bcl-2 targeting agents in addition to antihormonal therapy.
Figures
Figure 1
Principal component analysis and biplot. Projection of five-dimensional patient biomarker profiles with no missing data (439 instances) and the 439-dimensional biomarkers profiles onto the two leading principal components of a matrix consisting of expression profiles of Her2/neu, estrogen receptor (ER), progesterone receptor (PR), Bcl-2 and Bcl-2 antanogene-1 (Bag-1) present in all 439 samples. Each patient is represented by a distinct symbol (•, alive at 10 years; ×, dead at 10 years). The accumulated variation captured by the first and second principal components is 92% of the total variation. Overlaying a two-dimensional scatter plot representing the projection of the biomarkers ( ) onto the first and second principal components on top of the two-dimensional patient scatter plot representing the projection of their five-dimensional biomarker profiles onto the two leading principal components forms a biplot. The biplot can be used to read the approximated transformed expression levels.
) onto the first and second principal components on top of the two-dimensional patient scatter plot representing the projection of their five-dimensional biomarker profiles onto the two leading principal components forms a biplot. The biplot can be used to read the approximated transformed expression levels.
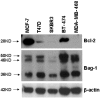
Figure 2
Expression of Bcl-2 antanogene-1 isoforms and Bcl-2. Expression of Bcl-2 antanogene-1 (Bag-1) isoforms and Bcl-2, using β-actin as a loading control in a panel of breast cancer cell lines.
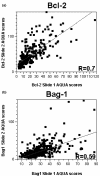
Figure 3
Regression plot for scores from breast cancer arrays stained for Bcl-2 and Bcl-2 antanogene-1. Regression plot for scores from the two breast cancer arrays stained for (a) Bcl-2 and (b) Bcl-2 antanogene-1 (Bag-1). AQUA, automated quantitative analysis.
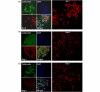
Figure 4
Immunoflourescent staining of Bcl-2 and Bcl-2 antanogene-1 in breast tumor tissue. (a) Cytoplasmic Bcl-2, (b) cytoplasmic and nuclear Bcl-2 antanogene-1 (Bag-1) and (c) nuclear Bag-1 staining in a breast cancer histospot – using cytokeratin to the define tumor mask, using 4',6-diamidino-2-phenylindole to define the nuclear compartment, and using Cy5 for identifying the target (Bcl-2 and Bag-1).
Figure 5
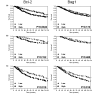
Kaplan–Meier survival curves for Bcl-2 and Bcl-2 antanogene-1. Kaplan–Meier survival curves for Bcl-2 and Bcl-2 antanogene-1 (Bag-1) automated quantitative analysis scores dichotomized by the median score for (a) the entire cohort of patients, (b) node-negative patients, and (c) node-positive patients.
Similar articles
- Clinical response after two cycles compared to HER2, Ki-67, p53, and bcl-2 in independently predicting a pathological complete response after preoperative chemotherapy in patients with operable carcinoma of the breast.
von Minckwitz G, Sinn HP, Raab G, Loibl S, Blohmer JU, Eidtmann H, Hilfrich J, Merkle E, Jackisch C, Costa SD, Caputo A, Kaufmann M; German Breast Group. von Minckwitz G, et al. Breast Cancer Res. 2008;10(2):R30. doi: 10.1186/bcr1989. Epub 2008 Apr 1. Breast Cancer Res. 2008. PMID: 18380893 Free PMC article. Clinical Trial. - BAG-1 expression correlates with Bcl-2, p53, differentiation, estrogen and progesterone receptors in invasive breast carcinoma.
Tang SC, Beck J, Murphy S, Chernenko G, Robb D, Watson P, Khalifa M. Tang SC, et al. Breast Cancer Res Treat. 2004 Apr;84(3):203-13. doi: 10.1023/B:BREA.0000019951.32001.93. Breast Cancer Res Treat. 2004. PMID: 15026618 - Absent progesterone receptor expression in the lymph node metastases of ER-positive, HER2-negative breast cancer is associated with relapse on tamoxifen.
Snell CE, Gough M, Middleton K, Hsieh M, Furnas L, Seidl B, Gibbons K, Pyke C, Shannon C, Woodward N, Armes JE. Snell CE, et al. J Clin Pathol. 2017 Nov;70(11):954-960. doi: 10.1136/jclinpath-2016-204304. Epub 2017 Apr 17. J Clin Pathol. 2017. PMID: 28416639 - Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues.
Cao L, Sun PL, Yao M, Jia M, Gao H. Cao L, et al. Hum Pathol. 2017 Oct;68:166-174. doi: 10.1016/j.humpath.2017.08.032. Epub 2017 Sep 9. Hum Pathol. 2017. PMID: 28899737 Review. - BAG-1 as a biomarker in early breast cancer prognosis: a systematic review with meta-analyses.
Papadakis ES, Reeves T, Robson NH, Maishman T, Packham G, Cutress RI. Papadakis ES, et al. Br J Cancer. 2017 Jun 6;116(12):1585-1594. doi: 10.1038/bjc.2017.130. Epub 2017 May 16. Br J Cancer. 2017. PMID: 28510570 Free PMC article. Review.
Cited by
- Prognostic value of bcl-2 expression among women with breast cancer in Libya.
Ermiah E, Buhmeida A, Khaled BR, Abdalla F, Salem N, Pyrhönen S, Collan Y. Ermiah E, et al. Tumour Biol. 2013 Jun;34(3):1569-78. doi: 10.1007/s13277-013-0687-1. Epub 2013 Feb 16. Tumour Biol. 2013. PMID: 23417836 - Does the expression of BCL2 have prognostic significance in malignant peritoneal mesothelioma?
Pillai K, Pourgholami MH, Chua TC, Morris DL. Pillai K, et al. Am J Cancer Res. 2013 Jun 20;3(3):312-22. Print 2013. Am J Cancer Res. 2013. PMID: 23841030 Free PMC article. - Differential expression of breast cancer-associated genes between stage- and age-matched tumor specimens from African- and Caucasian-American Women diagnosed with breast cancer.
Grunda JM, Steg AD, He Q, Steciuk MR, Byan-Parker S, Johnson MR, Grizzle WE. Grunda JM, et al. BMC Res Notes. 2012 May 22;5:248. doi: 10.1186/1756-0500-5-248. BMC Res Notes. 2012. PMID: 22616718 Free PMC article. - Choline plasmalogens isolated from swine liver inhibit hepatoma cell proliferation associated with caveolin-1/Akt signaling.
Zhan Y, Wang L, Liu J, Ma K, Liu C, Zhang Y, Zou W. Zhan Y, et al. PLoS One. 2013 Oct 15;8(10):e77387. doi: 10.1371/journal.pone.0077387. eCollection 2013. PLoS One. 2013. PMID: 24143228 Free PMC article. - Aspirin-induced Bcl-2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells.
Choi BH, Chakraborty G, Baek K, Yoon HS. Choi BH, et al. Exp Mol Med. 2013 Oct 11;45(10):e47. doi: 10.1038/emm.2013.91. Exp Mol Med. 2013. PMID: 24113271 Free PMC article.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. - DOI - PubMed
- Fisher B, Redmond C, Legault-Poisson S, Dimitrov NV, Brown AM, Wickerham DL, Wolmark N, Margolese RG, Bowman D, Glass AG. Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the National Surgical Adjuvant Breast and Bowel Project B-16. J Clin Oncol. 1990;8:1005–1018. - PubMed
- Binder C, Marx D, Overhoff R, Binder L, Schauer A, Hiddemann W. Bcl-2 protein expression in breast cancer in relation to established prognostic factors and other clinicopathological variables. Ann Oncol. 1995;6:1005–1010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous