Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer - PubMed (original) (raw)
Clinical Trial
. 2008 May 1;14(9):2710-6.
doi: 10.1158/1078-0432.CCR-07-4636.
Janice M Walshe, Douglas R Rosing, Neelima Denduluri, Arlene W Berman, Ujala Vatas, Margarita Velarde, Catherine K Chow, Seth M Steinberg, Diana Nguyen, Sherry X Yang, Sandra M Swain
Affiliations
- PMID: 18451236
- PMCID: PMC4406095
- DOI: 10.1158/1078-0432.CCR-07-4636
Clinical Trial
Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer
Chia C Portera et al. Clin Cancer Res. 2008.
Erratum in
- Clin Cancer Res. 2008 Jun 1;14(11):3641
Abstract
Purpose: To evaluate safety and efficacy of trastuzumab with pertuzumab in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer who had progressive disease on trastuzumab-based therapy.
Experimental design: Patients with measurable HER2(+) metastatic breast cancer, < or = 3 trastuzumab-based regimens, and left ventricular ejection fraction (LVEF) > or = 55% received 8 or 6 mg/kg trastuzumab and 840 mg pertuzumab i.v. followed by 6 mg/kg trastuzumab and 420 mg pertuzumab every 3 weeks. Cardiac evaluation and tumor response were assessed every 3 and 6 weeks, respectively.
Results: Eleven patients received 64 cycles of trastuzumab plus pertuzumab. A total of 92 echocardiograms and 8 cardiac magnetic resonance imaging studies were done. With the lower limit of normal LVEF 55%, left ventricular systolic dysfunction was observed in six patients, three grade 1, two grade 2, and one grade 3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events. The objective response rate was 18%. Two patients had partial responses, three had stable disease, and six had progressive disease. The median time to progression was 6 weeks. In baseline tumors from formalin-fixed paraffin-embedded primary and/or metastatic tumor biopsies, pHER2-Y1248 trended toward an increase in patients with partial response compared with those with stable disease/progressive disease (P = 0.095).
Conclusion: Trastuzumab plus pertuzumab may have clinical benefit in selected patients who have previously been treated with trastuzumab. Cardiac toxicity, although asymptomatic in most cases, was associated with this treatment. Further evaluation of efficacy of this combination is required to define the overall risks and benefits.
Figures
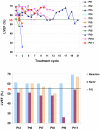
Figure 1
LVEF in all patients treated with trastuzumab combined with pertuzumab (A); LVEF in patients with LVSD (B) Abbreviations: LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; Pt, patient; C, cycle of treatment; F/U, follow up.
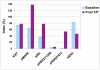
Figure 2
IHC staining indices of HER2 pathway markers and the percentage of Ki67-positive cells of a patient with progressive disease Abbreviations: IHC, immunohistochemistry; T/P, trastuzumab/pertuzumab.
Similar articles
- Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy.
Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, Fumoleau P, Gianni L. Baselga J, et al. J Clin Oncol. 2010 Mar 1;28(7):1138-44. doi: 10.1200/JCO.2009.24.2024. Epub 2010 Feb 1. J Clin Oncol. 2010. PMID: 20124182 Free PMC article. Clinical Trial. - Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer.
Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM; CLEOPATRA Study Group. Baselga J, et al. N Engl J Med. 2012 Jan 12;366(2):109-19. doi: 10.1056/NEJMoa1113216. Epub 2011 Dec 7. N Engl J Med. 2012. PMID: 22149875 Free PMC article. Clinical Trial. - Cardiac Safety of Dual Anti-HER2 Therapy in the Neoadjuvant Setting for Treatment of HER2-Positive Breast Cancer.
Yu AF, Singh JC, Wang R, Liu JE, Eaton A, Oeffinger KC, Steingart RM, Hudis CA, Dang CT. Yu AF, et al. Oncologist. 2017 Jun;22(6):642-647. doi: 10.1634/theoncologist.2016-0406. Epub 2017 Mar 24. Oncologist. 2017. PMID: 28341761 Free PMC article. - Effect of pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer: A meta-analysis .
Tian T, Ye J, Zhou S. Tian T, et al. Int J Clin Pharmacol Ther. 2017 Sep;55(9):720-727. doi: 10.5414/CP202921. Int J Clin Pharmacol Ther. 2017. PMID: 28737130 - A systematic review of dual targeting in HER2-positive breast cancer.
Kümler I, Tuxen MK, Nielsen DL. Kümler I, et al. Cancer Treat Rev. 2014 Mar;40(2):259-70. doi: 10.1016/j.ctrv.2013.09.002. Epub 2013 Sep 11. Cancer Treat Rev. 2014. PMID: 24080156 Review.
Cited by
- Molecular Mechanisms of Trastuzumab Resistance in HER2 Overexpressing Breast Cancer.
Fiszman GL, Jasnis MA. Fiszman GL, et al. Int J Breast Cancer. 2011;2011:352182. doi: 10.4061/2011/352182. Epub 2011 Sep 6. Int J Breast Cancer. 2011. PMID: 22295219 Free PMC article. - Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer.
Krop IE, Modi S, LoRusso PM, Pegram M, Guardino E, Althaus B, Lu D, Strasak A, Elias A. Krop IE, et al. Breast Cancer Res. 2016 Mar 15;18(1):34. doi: 10.1186/s13058-016-0691-7. Breast Cancer Res. 2016. PMID: 26979312 Free PMC article. Clinical Trial. - Management of breast cancer with targeted agents: importance of heterogeneity. [corrected].
Di Cosimo S, Baselga J. Di Cosimo S, et al. Nat Rev Clin Oncol. 2010 Mar;7(3):139-47. doi: 10.1038/nrclinonc.2009.234. Epub 2010 Feb 2. Nat Rev Clin Oncol. 2010. PMID: 20125090 Review. - Present and future evolution of advanced breast cancer therapy.
Alvarez RH. Alvarez RH. Breast Cancer Res. 2010;12 Suppl 2(Suppl 2):S1. doi: 10.1186/bcr2572. Epub 2010 Oct 22. Breast Cancer Res. 2010. PMID: 21050422 Free PMC article. Review. - Which patients with metastatic breast cancer benefit from subsequent lines of treatment? An update for clinicians.
Palumbo R, Sottotetti F, Riccardi A, Teragni C, Pozzi E, Quaquarini E, Tagliaferri B, Bernardo A. Palumbo R, et al. Ther Adv Med Oncol. 2013 Nov;5(6):334-50. doi: 10.1177/1758834013508197. Ther Adv Med Oncol. 2013. PMID: 24179488 Free PMC article.
References
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001(344):783–92. - PubMed
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005(353):1673–84. - PubMed
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. - PubMed
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. - PubMed
- Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous