Ribosomal RNAs are tolerant toward genetic insertions: evolutionary origin of the expansion segments - PubMed (original) (raw)
Ribosomal RNAs are tolerant toward genetic insertions: evolutionary origin of the expansion segments
Takeshi Yokoyama et al. Nucleic Acids Res. 2008 Jun.
Abstract
Ribosomal RNAs (rRNAs), assisted by ribosomal proteins, form the basic structure of the ribosome, and play critical roles in protein synthesis. Compared to prokaryotic ribosomes, eukaryotic ribosomes contain elongated rRNAs with several expansion segments and larger numbers of ribosomal proteins. To investigate architectural evolution and functional capability of rRNAs, we employed a Tn5 transposon system to develop a systematic genetic insertion of an RNA segment 31 nt in length into Escherichia coli rRNAs. From the plasmid library harboring a single rRNA operon containing random insertions, we isolated surviving clones bearing rRNAs with functional insertions that enabled rescue of the E. coli strain (Delta7rrn) in which all chromosomal rRNA operons were depleted. We identified 51 sites with functional insertions, 16 sites in 16S rRNA and 35 sites in 23S rRNA, revealing the architecture of E. coli rRNAs to be substantially flexible. Most of the insertion sites show clear tendency to coincide with the regions of the expansion segments found in eukaryotic rRNAs, implying that eukaryotic rRNAs evolved from prokaryotic rRNAs suffering genetic insertions and selections.
Figures
Figure 1.
Outline of the systematic genetic insertion of RNA segment in rRNAs. (A) A Tn5 transposon carrying a DHFR marker flanked by two MMEs was randomly inserted in vitro into a plasmid pRB102 harboring the _rrn_B operon, to construct the insertion library. Escherichia coli DH5α was transformed with the library and selected using trimethoprim and kanamycin. About 10 000 colonies were scraped together. To prepare the plasmid mixture, the DHFR marker in each plasmid was removed by Bsp1407I digestion. After re-ligation, the 31-base inserted RNA segment remained at the insertion site. (B) Detailed description of the site of insertion. MME contains a Bsp1407I site. At the sites of insertion, nine (or 10) bases of the target sequences are duplicated as a result of Tn5 transposition. It is just described as a ‘duplicated site’. The 31-nt inserted sequence, composed of residual linker sequence (22 nt) and duplicated sequence (9 or 10 nt), makes a short helix in the rRNA sequence.
Figure 2.
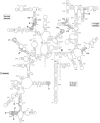
Sites of functional insertions on the secondary structure of E. coli 16S rRNA. The duplicated sequences at the sites of the 16 functional insertions (i1–i16) are shown by open ellipses. Numbering of the sites is described in Table 1. Helices (h1–h45) in 16S rRNA are numbered.
Figure 3.
Sites of functional insertions on the secondary structure of E. coli 23S rRNA. The duplicated sequences at the sites of the 35 functional insertions (I1–I35) are shown by open ellipses. Numbering of the sites is described in Table 2. Helices (H1–H101) in 23S rRNA are numbered.
Figure 4.
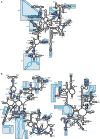
Sites of functional insertions mapped on the tertiary structure of E. coli ribosomes. The sites of insertions are marked in blue on the E. coli 30S (upper panels) and 50S (lower panels) subunits, showing numbering of insertions. The left and right figures represent the solvent side and the interface, respectively. RNAs and proteins are shown in light gray and dark gray, respectively. Ribosomal proteins S16, L4 and L15 are indicated. The atomic coordinates of each subunit were obtained from the Protein Data Bank (code 2AW4 and 2AVY) (5). The three-dimensional structures were displayed using Ras Top version 2.0.3.
Figure 5.
Secondary structure diagrams of E. coli rRNAs superimposed with S. cerevisiae rRNAs, indicating the sites of functional insertions. Escherichia coli 16S (A) and 23S (B) rRNAs are shown as black lines. Saccharomyces cerevisiae 18S (A) and 25S (B) rRNAs are shown as blue lines. Expansion segments are boxed and shaded in blue. The sites of functional insertions are mapped on the secondary structures of rRNAs using numbered blue circles.
Similar articles
- Systematic deletion of rRNAs for investigating ribosome architecture and function.
Kitahara K, Sato NS, Namba N, Yokota T, Tsujimura T, Suzuki T. Kitahara K, et al. Nucleic Acids Symp Ser (Oxf). 2006;(50):287-8. doi: 10.1093/nass/nrl143. Nucleic Acids Symp Ser (Oxf). 2006. PMID: 17150930 - rRNA mutations that inhibit transfer-messenger RNA activity on stalled ribosomes.
Crandall J, Rodriguez-Lopez M, Pfeiffer M, Mortensen B, Buskirk A. Crandall J, et al. J Bacteriol. 2010 Jan;192(2):553-9. doi: 10.1128/JB.01178-09. Epub 2009 Nov 6. J Bacteriol. 2010. PMID: 19897649 Free PMC article. - Structural and evolutionary insights into ribosomal RNA methylation.
Sergiev PV, Aleksashin NA, Chugunova AA, Polikanov YS, Dontsova OA. Sergiev PV, et al. Nat Chem Biol. 2018 Feb 14;14(3):226-235. doi: 10.1038/nchembio.2569. Nat Chem Biol. 2018. PMID: 29443970 Review. - RNA-protein interactions in the Escherichia coli ribosome.
Brimacombe R. Brimacombe R. Biochimie. 1991 Jul-Aug;73(7-8):927-36. doi: 10.1016/0300-9084(91)90134-m. Biochimie. 1991. PMID: 1720671 Review.
Cited by
- Computing the origin and evolution of the ribosome from its structure - Uncovering processes of macromolecular accretion benefiting synthetic biology.
Caetano-Anollés G, Caetano-Anollés D. Caetano-Anollés G, et al. Comput Struct Biotechnol J. 2015 Jul 26;13:427-47. doi: 10.1016/j.csbj.2015.07.003. eCollection 2015. Comput Struct Biotechnol J. 2015. PMID: 27096056 Free PMC article. Review. - The Jigsaw Puzzle of mRNA Translation Initiation in Eukaryotes: A Decade of Structures Unraveling the Mechanics of the Process.
Hashem Y, Frank J. Hashem Y, et al. Annu Rev Biophys. 2018 May 20;47:125-151. doi: 10.1146/annurev-biophys-070816-034034. Epub 2018 Mar 1. Annu Rev Biophys. 2018. PMID: 29494255 Free PMC article. - Cryo-electron microscopy visualization of a large insertion in the 5S ribosomal RNA of the extremely halophilic archaeon Halococcus morrhuae.
Tirumalai MR, Kaelber JT, Park DR, Tran Q, Fox GE. Tirumalai MR, et al. FEBS Open Bio. 2020 Oct;10(10):1938-1946. doi: 10.1002/2211-5463.12962. Epub 2020 Sep 17. FEBS Open Bio. 2020. PMID: 32865340 Free PMC article. - One step engineering of the small-subunit ribosomal RNA using CRISPR/Cas9.
Kannan K, Tsvetanova B, Chuang RY, Noskov VN, Assad-Garcia N, Ma L, Hutchison Iii CA, Smith HO, Glass JI, Merryman C, Venter JC, Gibson DG. Kannan K, et al. Sci Rep. 2016 Aug 4;6:30714. doi: 10.1038/srep30714. Sci Rep. 2016. PMID: 27489041 Free PMC article. - Evolutionary triplet models of structured RNA.
Bradley RK, Holmes I. Bradley RK, et al. PLoS Comput Biol. 2009 Aug;5(8):e1000483. doi: 10.1371/journal.pcbi.1000483. Epub 2009 Aug 28. PLoS Comput Biol. 2009. PMID: 19714212 Free PMC article.
References
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. - PubMed
- Wimberly BT, Brodersen DE, Clemons W.M., Jr., Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. - PubMed
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292:883–896. - PubMed
- Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, et al. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell. 2000;102:615–623. - PubMed
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials