Rapid signal transduction in living cells is a unique feature of mechanotransduction - PubMed (original) (raw)
Rapid signal transduction in living cells is a unique feature of mechanotransduction
Sungsoo Na et al. Proc Natl Acad Sci U S A. 2008.
Abstract
It is widely postulated that mechanotransduction is initiated at the local force-membrane interface by inducing local conformational changes of proteins, similar to soluble ligand-induced signal transduction. However, all published reports are limited in time scale to address this fundamental issue. Using a FRET-based cytosolic Src reporter in a living cell, we quantified changes of Src activities as a local stress via activated integrins was applied. The stress induced rapid (<0.3 s) activation of Src at remote cytoplasmic sites, which depends on the cytoskeletal prestress. In contrast, there was no Src activation within 12 s of soluble epidermal growth factor (EGF) stimulation. A 1.8-Pa stress over a focal adhesion activated Src to the same extent as 0.4 ng/ml EGF at long times (minutes), and the energy levels for mechanical stimulation and chemical stimulation were comparable. The effect of both stress and EGF was less than additive. Nanometer-scale cytoskeletal deformation analyses revealed that the strong activation sites of Src by stress colocalized with large deformation sites of microtubules, suggesting that microtubules are essential structures for transmitting stresses to activate cytoplasmic proteins. These results demonstrate that rapid signal transduction via the prestressed cytoskeleton is a unique feature of mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
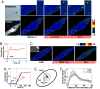
Fig. 1.
Rapid Src activation in response to localized mechanical stress. (A) A 4.5-μm RGD-coated ferromagnetic bead was attached to the apical surface of the cell (Left Upper, black dot is the bead) for 15 min to allow integrin clustering and formation of focal adhesions around the bead (38). Bead binding alone induced little Src activation (
Fig. S9
). The bead was magnetized horizontally and subjected to a vertical magnetic field (step function) that applies a mechanical stress σ (specific torque = 17.5 Pa) to the cell. A genetically encoded, CFP-YFP cytosolic Src reporter was transfected into the smooth muscle cells by following published procedures (7). The cytosolic Src reporter was uniformly distributed in the cytoplasm (Left Lower, YFP). The stress application induced rapid changes (<0.3 s) in FRET of the Src reporter at discrete, distant sites in the cytoplasm (see Insets) (focal plane is ≈1 μm above cell base), indicating rapid Src activation (
Fig. S1
). Images are scaled, and regions of large FRET changes (strong Src activity) are shown in red. The black arrow indicates bead movement direction. (Scale bar, 10 μm.) (B) Time course of normalized CFP/YFP emission ratio, an index of Src activation in response to mechanical or soluble growth factor EGF stimulation. n = 12 cells for +σ; n = 8 cells for +EGF. Error bars represent SEM. (C) Time course of CFP/YFP emission ratio in response to EGF in a representative cell (see
Fig. S2
for full time course). EGF was locally released on top of the cell apical surface (<1 μm above) by using a micropipette (25 μm in diameter; top right of the Inset) (Scale bar, 20 μm) that was controlled by a micromanipulator and CellTram Vario. EGF (50 ng/ml) was released at a flow rate of 2 × 104 μm3/ms continuously for 5 min. Because the diffusion coefficient of a protein in water is ≈100 μm2/s (39), it takes ≈10 ms for EGF to reach the cell apical surface, and local EGF concentration at the cell apical surface is ≈40 ng/ml. (D) Time course of average Src activation from eight different cells after EGF treatment as in C. Error bars represent SEM. (E) Src activation at different cytoplasmic sites. At every 1 μm away from the bead, the emission ratio image after mechanical stimulation was compared pixel-by-pixel with that before mechanical stimulation. (F) The number of activated pixels (percentage of total activated pixels) at a given time versus distance from the bead after 0.3 s and 2.7 s of mechanical stimulation (see
Fig. S4
with 90% threshold). Maximum number of Src activation was observed at ≈15 μm away from the bead. Note that the spatial distribution of Src activation but not the intensity of Src activation is summarized here. n = 8 cells. Error bars represent SEM.
Fig. 2.
Src activation depends on stress probe specificity, substrate rigidity, intact F-actin, and prestress. (A) Probe specificity on Src activation. Mechanical stimulation via the magnetic bead coated with RGD or anti-β1-activating antibody (P4G11), induced Src activation in the cytoplasm but not anti-β1-nonactivating antibody (K20), AcLDL (binds scavenger receptors), or PLL (strong nonspecific surface binding) (
Fig. S5
). RGD, n = 12 cells; P4G11, n = 4 cells; K20, n = 3 cells; AcLDL, n = 4 cells; PLL, n = 3 cells. Error bars represent SEM. (B) Mechanical stimulation of cells plated on soft (0.3 kPa, n = 10 cells) polyacrylamide gel substrate did not induce Src activation, whereas the cell on relatively hard (8 kPa, n = 4 cells) substrate induced strong Src activation (red arrows). Cells on PLL substrate (n = 8 cells) that do not form basal focal adhesions and stress fibers (12) did not activate Src in response to mechanical stress. White arrows indicate magnetic bead movement direction (stress = 17.5 Pa). (Scale bars, 10 μm.) (C) Preincubating cells with CytoD (1 μg/ml for 15 min; n = 5 cells), LatA (1 μM for 15 min; n = 4 cells) to disrupt actin microfilaments, Bleb (50 μM for 20 min; n = 5 cells) to inhibit myosin II, or DBcAMP (1 mM for 15 min; n = 4 cells) to relax the cell, prevented stress-induced Src activation (
Fig. S6
).
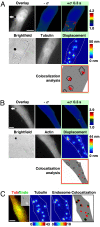
Fig. 3.
Rapid, long-range strong Src activation sites in the cytoplasm colocalize with sites of large microtubule displacements. (A) The cell was cotransfected with CFP-YFP Src reporter and mCherry-tubulin. A step function stress (17.5 Pa) was first applied for 3 s via an RGD-coated bead, and FRET changes were recorded. Then the microtubule deformation map was acquired when an oscillatory stress was applied for ≈30 s (0.3 Hz; peak stress = 24.5 Pa, equivalent to a constant stress of 17.5 Pa) (12). In this representative cell, strong Src activation sites coincide with large deformation sites (>15 nm) of microtubules in the same cell at the same focal plane (≈1 μm above cell base). The overlay image is the YFP Src reporter image superimposed with the bead. Pink circles indicate bead center position; white arrows represent microtubule deformation direction. Red arrows point to strong Src activation sites. In the colocalization analysis panel, red represents strong Src activation, and black lines represent large microtubule displacements. Three other different cells showed similar results. Of strong Src activation sites, ≈80% (15 of 19) were colocalized with sites of microtubule deformation >15 nm. (Scale bar, 10 μm.) (B) Src activation sites do not colocalize with F-actin deformation sites. The cell was cotransfected with CFP-YFP Src reporter and mCherry-actin. A step function stress (17.5 Pa) was first applied for 3 s via an RGD-coated bead, and FRET changes were recorded. Then the actin deformation map was acquired when an oscillatory stress was applied in the same way as in A. In contrast to A, strong Src activation sites do not coincide with large deformation sites (>15 nm) of actin in the same cell at the same focal plane (≈1 μm above cell base). Pink circles indicate bead center position; white arrows represent actin deformation direction. In the colocalization analysis panel, red represents strong Src activation, and black lines represent large actin displacements >15 nm. Five other different cells showed similar results. Of strong Src activation sites, only ≈12% (3 of 26) were colocalized with sites of actin deformation >15 nm. (Scale bar, 10 μm.) (C) Large microtubule deformation sites colocalize with endosomal membrane deformation in the same cell at the same focal plane (≈1 μm above cell base). The Inset is the bright-field image of the cell. The cell was cotransfected with mCherry-tubulin and pAcGFP1-endo (Left). The displacement maps of microtubule and endosome were acquired when an oscillatory stress was applied separately (30 s each) (peak stress = 24.5 Pa, frequency = 0.3 Hz). In the colocalization panel, red represents microtubule displacements >15 nm, green represents endosome displacements >8 nm, and overlapped black shows colocalization sites of microtubule and endosome. Of 13 large endosome displacement sites in three different cells, 10 were colocalized (≈80%) with large microtubule deformation sites. The color bar unit of the displacement map is in nanometers. (Scale bar, 10 μm.)
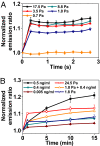
Fig. 4.
Src activity in response to mechanical and/or EGF stimulation at long times. (A) Src activation is stress-magnitude dependent. n = 12 cells for stress of 17.5 Pa; 4 for 8.8 Pa; 4 for 3.5 Pa; 4 for 1.8 Pa; 3 for 0.7 Pa. It appears that Src is activated when the applied stress is >1.8 Pa. Error bars represent SEM. (B) Stress magnitude equivalent of EGF concentration. A 1.8-Pa oscillatory stress activated Src to the same extent as 0.4 ng/ml EGF (final global concentration). Stress together with EGF further increased Src activation by ≈40% at 15 min, suggesting less than additive effects. P values are 0.027, 0.036, and 0.005 between 1.8 Pa + 0.4 ng/ml and 0.4 ng/ml for 5, 10, and 15 min; P values are 0.024, 0.021, and 0.047 between 1.8 Pa + 0.4 ng/ml and 1.8 Pa for 5, 10, and 15 min. n = 4 cells for 0.5 ng/ml; 3 for 0.4 ng/ml; 3 for 0.005 ng/ml; 3 for 24.5 Pa; 7 for 1.8 Pa + 0.4 ng/ml; 6 for 1.8 Pa. Error bars represent SEM.
Similar articles
- Epidermal growth factor-induced activation and translocation of c-Src to the cytoskeleton depends on the actin binding domain of the EGF-receptor.
Van der Heyden MA, Oude Weernink PA, Van Oirschot BA, Van Bergen en Henegouwen PM, Boonstra J, Rijksen G. Van der Heyden MA, et al. Biochim Biophys Acta. 1997 Dec 12;1359(3):211-21. doi: 10.1016/s0167-4889(97)00105-5. Biochim Biophys Acta. 1997. PMID: 9434127 - Visualizing the mechanical activation of Src.
Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Wang Y, et al. Nature. 2005 Apr 21;434(7036):1040-5. doi: 10.1038/nature03469. Nature. 2005. PMID: 15846350 - c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation.
Chang JH, Gill S, Settleman J, Parsons SJ. Chang JH, et al. J Cell Biol. 1995 Jul;130(2):355-68. doi: 10.1083/jcb.130.2.355. J Cell Biol. 1995. PMID: 7542246 Free PMC article. - Role of c-Src tyrosine kinase in EGF-induced mitogenesis.
Belsches AP, Haskell MD, Parsons SJ. Belsches AP, et al. Front Biosci. 1997 Oct 15;2:d501-18. doi: 10.2741/a208. Front Biosci. 1997. PMID: 9331427 Review. - Invadosome regulation by adhesion signaling.
Destaing O, Block MR, Planus E, Albiges-Rizo C. Destaing O, et al. Curr Opin Cell Biol. 2011 Oct;23(5):597-606. doi: 10.1016/j.ceb.2011.04.002. Epub 2011 May 6. Curr Opin Cell Biol. 2011. PMID: 21550788 Review.
Cited by
- Vinculin tension distributions of individual stress fibers within cell-matrix adhesions.
Chang CW, Kumar S. Chang CW, et al. J Cell Sci. 2013 Jul 15;126(Pt 14):3021-30. doi: 10.1242/jcs.119032. Epub 2013 May 17. J Cell Sci. 2013. PMID: 23687380 Free PMC article. - Interplay between cytoskeletal stresses and cell adaptation under chronic flow.
Verma D, Ye N, Meng F, Sachs F, Rahimzadeh J, Hua SZ. Verma D, et al. PLoS One. 2012;7(9):e44167. doi: 10.1371/journal.pone.0044167. Epub 2012 Sep 19. PLoS One. 2012. PMID: 23028495 Free PMC article. - Molecular force transduction by ion channels: diversity and unifying principles.
Sukharev S, Sachs F. Sukharev S, et al. J Cell Sci. 2012 Jul 1;125(Pt 13):3075-83. doi: 10.1242/jcs.092353. Epub 2012 Jul 13. J Cell Sci. 2012. PMID: 22797911 Free PMC article. Review. - Finding the weakest link: exploring integrin-mediated mechanical molecular pathways.
Roca-Cusachs P, Iskratsch T, Sheetz MP. Roca-Cusachs P, et al. J Cell Sci. 2012 Jul 1;125(Pt 13):3025-38. doi: 10.1242/jcs.095794. Epub 2012 Jul 13. J Cell Sci. 2012. PMID: 22797926 Free PMC article. Review. - Mechanosensitivity of a rapid bioluminescence reporter system assessed by atomic force microscopy.
Tesson B, Latz MI. Tesson B, et al. Biophys J. 2015 Mar 24;108(6):1341-1351. doi: 10.1016/j.bpj.2015.02.009. Biophys J. 2015. PMID: 25809248 Free PMC article.
References
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. - PubMed
- Janmey PA, McCulloch CA. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. - PubMed
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. - PubMed
- Ingber DE. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006;20:811–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous