Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer's disease - PubMed (original) (raw)
Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer's disease
William J Meilandt et al. J Neurosci. 2008.
Abstract
The enkephalin signaling pathway regulates various neural functions and can be altered by neurodegenerative disorders. In Alzheimer's disease (AD), elevated enkephalin levels may reflect compensatory processes or contribute to cognitive impairments. To differentiate between these possibilities, we studied transgenic mice that express human amyloid precursor protein (hAPP) and amyloid-beta (Abeta) peptides in neurons and exhibit key aspects of AD. Met-enkephalin levels in neuronal projections from the entorhinal cortex and dentate gyrus (brain regions important for memory that are affected in early stages of AD) were increased in hAPP mice, as were preproenkephalin mRNA levels. Genetic manipulations that exacerbate or prevent excitotoxicity also exacerbated or prevented the enkephalin alterations. In human AD brains, enkephalin levels in the dentate gyrus were also increased. In hAPP mice, enkephalin elevations correlated with the extent of Abeta-dependent neuronal and behavioral alterations, and memory deficits were reduced by irreversible blockade of mu-opioid receptors with the antagonist beta-funaltrexamine. We conclude that enkephalin elevations may contribute to cognitive impairments in hAPP mice and possibly in humans with AD. The therapeutic potential of reducing enkephalin production or signaling merits further exploration.
Figures
Figure 1.
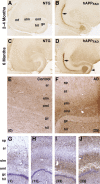
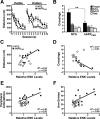
Increased hippocampal ENK immunoreactivity in hAPPFAD mice and humans with AD. A–D, Horizontal brain sections of 2- to 4-month-old (A, B) and 6-month-old (C, D) mice were immunostained for ENK. Compared with NTG controls (A, C), hAPPFAD mice (B, D) had increased ENK immunoreactivity in the outer molecular layer (arrowhead) and hilar region of the dentate gyrus, the stratum lacunosum-moleculare, and the mossy fibers (arrows). E, F, Horizontal sections of human hippocampal tissue depicting differences in ENK immunoreactivity in a 71-year-old nondemented control (E) and a 90-year-old AD patient (F). G–J, Hippocampal sections from different AD cases were immunostained for ENK and counterstained with hematoxylin. Blessed Dementia Scale scores are shown in parentheses. gc, Granular layer; hil, hilus; mf, mossy fibers; oml, outer molecular layer; slm, stratum lacunosum-moleculare; sp, stratum pyramidale; sr, stratum radiatum. Abbreviations are the same in subsequent figures.
Figure 2.
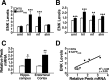
Quantitative comparison of hippocampal ENK and Penk expression in hAPPFAD mice and NTG controls. A, B, Densitometric quantitation of ENK immunoreactivity in specific hippocampal subregions (normalized to values from the stratum radiatum of CA1) from sections of 2- to 4-month-old (A) and 6-month-old (B) mice. n = 10–14 mice per age and genotype. C, Quantitative RT-PCR analysis of Penk mRNA levels in the hippocampus of 2- to 3-month-old mice and in the entorhinal cortex of 4- to 6-month-old mice relative to NTG controls. n = 6 (hippocampus) or 11–15 (entorhinal cortex) mice per age and genotype. D, In 2- to 3-month-old hAPPFAD mice, ENK immunoreactivity levels in the hilus correlated with hippocampal Penk mRNA levels. _R_2 and p values refer to hAPPFAD mice only. *p < 0.05, **p < 0.01, ***p < 0.001 versus NTG (Student's t test). Values are mean ± SEM.
Figure 3.
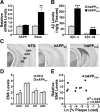
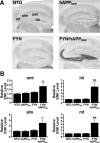
Comparable expression of wild-type hAPP does not increase hippocampal ENK and Penk expression. A, B, Hippocampal levels of hAPP mRNA (A right), Penk mRNA (A, left), Aβ1–x (B, left), and Aβ1–42 (B, right) in hAPPWT mice from line I5 and hAPPFAD mice from line J20 (n = 7–10 mice per genotype; age, 2–3 months). C, Coronal sections illustrating typical ENK immunostaining in 4- to 6-month-old mice of the indicated genotypes. D, Densitometric quantitation of ENK immunoreactivity revealed no significant differences between hAPPWT mice and age-matched NTG controls in all hippocampal subfields except the oml of the dentate gyrus. E, Levels of ENK immunoreactivity in the hilus did not correlate with hippocampal plaque loads in 6-month-old hAPPFAD mice. *p < 0.05, **p < 0.01, ***p < 0.001 (Student's t test). Values are mean ± SEM.
Figure 4.
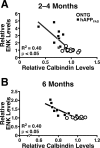
ENK elevations in hAPPFAD mice correlate with calbindin depletions in the dentate gyrus. A, B, Horizontal brain sections of 2- to 4-month-old (A) and 6-month-old (B) hAPPFAD mice (J20 line) were immunostained for ENK and calbindin, and immunoreactivities were quantitated by densitometry. ENK elevations in the hilus correlated with calbindin depletions in the outer molecular layer in hAPPFAD mice. _R_2 and p values refer to hAPPFAD mice only.
Figure 5.
ENK elevations correlate with behavioral alterations in hAPPFAD mice. A, B, hAPPFAD mice from line J20 and NTG controls (n = 10 mice per genotype; age, 3–5 months) were trained in a Morris water maze. A, No differences were found on the first training trial (T1). However, hAPPFAD mice showed learning deficits in the cued-platform (p < 0.05) and hidden-platform (p = 0.001) components of this task (repeated-measures ANOVA). B, During a probe trial (platform removed) at the beginning of the fourth day of training (before session 7), hAPPFAD mice crossed over the target location less often than NTG controls. C–F, ENK elevations in the hilus of hAPPFAD mice correlated with increased average distance required to reach the hidden platform (sessions 1–10) in the water maze (C), decreased target crossings during the probe trial (D), and hyperactivity in the open field (E) and Y-maze (F). _R_2 and p values refer to hAPPFAD mice only. **p < 0.01 (Student's t test). Values are mean ± SEM.
Figure 6.
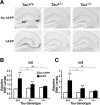
Increased neuronal Fyn expression elicits ENK elevations in low-expresser hAPPFAD mice. hAPPFAD mice from the low-expresser J9 line (hAPPlow) were crossed with mice that overexpress murine Fyn in neurons (FYN). The resulting offspring were analyzed at 7–9 months of age. A, Sagittal sections of the hippocampus immunostained for ENK. B, Densitometric quantitation of ENK immunoreactivity in different subfields of the hippocampus (n = 6–8 mice per genotype). Two-way ANOVA revealed a significant APP × FYN interaction (p < 0.01) for both the hilus and mossy fibers. *p < 0.05, **p < 0.01 versus NTG (Dunnett's test). Values are mean ± SEM.
Figure 7.
Tau reduction prevents ENK elevations in hAPPFAD mice from line J20. Mice were analyzed at 5–7 months of age. A, Coronal sections of the hippocampus immunostained for ENK. B, C, ENK immunoreactivity in the hilus (B) and mossy fibers (C) was quantitated densitometrically in the groups indicated [n = 7–10 mice per genotype, except for hAPPFAD/Tau+/+ mice (n = 3)]. Two-way ANOVA revealed a significant APP effect (p < 0.001), Tau effect (p < 0.05), and APP × Tau interaction (p < 0.01) for both regions. **p < 0.01 versus Tau+/+ mice without hAPP (Dunnett's test); +p < 0.05, ++p < 0.01 versus Tau+/+ mice with hAPP (Fisher's PLSD test). Values are mean ± SEM.
Figure 8.
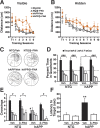
μ-Opioid receptor antagonist ameliorates Aβ-induced behavioral deficits in hAPPFAD mice. hAPPFAD mice and NTG controls were treated with the irreversible μ-opioid receptor antagonist β-FNA or vehicle (Veh) and tested in the Morris water maze (n = 7–9 mice per genotype and treatment; age, 9–10 months). A, B, β-FNA improved the ability of hAPPFAD mice to learn the cued-platform location (p < 0.01 for genotype × treatment interaction by ANOVA; A), but not the hidden-platform location (B). The β-FNA regimen used here had no effect on NTG controls. C–F, Results from a probe trial performed 72 h after the last hidden-platform training trial. C, Representative swim paths show increased preference for the target quadrant in β-FNA-treated hAPPFAD mice. D, Percentage of time spent in different quadrants (p < 0.05 for group × quadrant interaction by ANOVA). E, Number of times mice crossed the platform location or corresponding locations in the other quadrants (p < 0.05 for group × quadrant interaction by ANOVA). F, Latency to reach the platform location (p < 0.05 for genotype × treatment interaction by ANOVA). ###p < 0.001, #p < 0.05 versus all nontarget quadrants; *p < 0.05, **p < 0.01 versus all other groups (Fisher's PLSD test); +p < 0.05 versus β-FNA-treated hAPP mice (Student's t test). Values are mean ± SEM.
Similar articles
- Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease.
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Palop JJ, et al. Neuron. 2007 Sep 6;55(5):697-711. doi: 10.1016/j.neuron.2007.07.025. Neuron. 2007. PMID: 17785178 Free PMC article. - Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits.
Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Palop JJ, et al. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9572-7. doi: 10.1073/pnas.1133381100. Epub 2003 Jul 24. Proc Natl Acad Sci U S A. 2003. PMID: 12881482 Free PMC article. - Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer's disease.
Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, Bender A, Mucke L. Chin J, et al. J Neurosci. 2007 Mar 14;27(11):2727-33. doi: 10.1523/JNEUROSCI.3758-06.2007. J Neurosci. 2007. PMID: 17360894 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Epilepsy and cognitive impairments in Alzheimer disease.
Palop JJ, Mucke L. Palop JJ, et al. Arch Neurol. 2009 Apr;66(4):435-40. doi: 10.1001/archneurol.2009.15. Epub 2009 Feb 9. Arch Neurol. 2009. PMID: 19204149 Free PMC article. Review.
Cited by
- Memory-enhancing effect of aspirin is mediated through opioid system modulation in an AlCl3-induced neurotoxicity mouse model.
Rizwan S, Idrees A, Ashraf M, Ahmed T. Rizwan S, et al. Exp Ther Med. 2016 May;11(5):1961-1970. doi: 10.3892/etm.2016.3147. Epub 2016 Mar 11. Exp Ther Med. 2016. PMID: 27168835 Free PMC article. - Descriptive molecular pharmacology of the δ opioid receptor (DOR): A computational study with structural approach.
Goode-Romero G, Dominguez L. Goode-Romero G, et al. PLoS One. 2024 Jul 11;19(7):e0304068. doi: 10.1371/journal.pone.0304068. eCollection 2024. PLoS One. 2024. PMID: 38991032 Free PMC article. - Network abnormalities and interneuron dysfunction in Alzheimer disease.
Palop JJ, Mucke L. Palop JJ, et al. Nat Rev Neurosci. 2016 Dec;17(12):777-792. doi: 10.1038/nrn.2016.141. Epub 2016 Nov 10. Nat Rev Neurosci. 2016. PMID: 27829687 Free PMC article. Review. - Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer's disease model mice.
Woodling NS, Colas D, Wang Q, Minhas P, Panchal M, Liang X, Mhatre SD, Brown H, Ko N, Zagol-Ikapitte I, van der Hart M, Khroyan TV, Chuluun B, Priyam PG, Milne GL, Rassoulpour A, Boutaud O, Manning-Boğ AB, Heller HC, Andreasson KI. Woodling NS, et al. Brain. 2016 Jul;139(Pt 7):2063-81. doi: 10.1093/brain/aww117. Epub 2016 May 13. Brain. 2016. PMID: 27190010 Free PMC article. - Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks.
Palop JJ, Mucke L. Palop JJ, et al. Nat Neurosci. 2010 Jul;13(7):812-8. doi: 10.1038/nn.2583. Nat Neurosci. 2010. PMID: 20581818 Free PMC article. Review.
References
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. - PubMed
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. - PubMed
- Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2004. Peptides. 2005;26:2629–2711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG023501/AG/NIA NIH HHS/United States
- R01 NS041787-08/NS/NINDS NIH HHS/United States
- NS041787/NS/NINDS NIH HHS/United States
- RR018928/RR/NCRR NIH HHS/United States
- C06 RR018928-01/RR/NCRR NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- AG023501/AG/NIA NIH HHS/United States
- C06 RR018928/RR/NCRR NIH HHS/United States
- R01 NS041787/NS/NINDS NIH HHS/United States
- K08 NS054811-03/NS/NINDS NIH HHS/United States
- P01 AG022074-06/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- K08 NS054811/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous