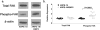FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction - PubMed (original) (raw)
FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction
Shawn A Morales et al. Invest Ophthalmol Vis Sci. 2009 Jan.
Abstract
Purpose: Proliferative vitreoretinopathy (PVR) occurs in approximately 10% of patients after retinal detachment. PVR results from a multiphase process that leads to an aberrant wound-healing strategy with contractile cellular forces and tractional retinal detachment (TRD). Epithelial membrane protein (EMP) 2 controls cell surface expression and function of integrin isoforms associated with cellular contraction in many cell types. Since EMP2 is highly expressed in retinal pigment epithelium, this study investigates the role of EMP2 in collagen gel contraction.
Methods: EMP2 expression was recombinantly modified in the ARPE-19 cell line. Cell surface integrin expression was assessed by flow cytometry. Collagen gel contraction was assessed by using an in vitro assay and the percentage of contraction was quantified. Proliferation and migration were measured by BrdU incorporation and a wound-healing assay, respectively. Cellular invasion was investigated with polycarbonate membranes coated with collagen.
Results: EMP2 expression levels correlated positively with the ability to contract collagen gels. Compared with wild-type ARPE-19 cells, the cells with increased EMP2 expression exhibited enhanced contraction (P = 0.02), and decreased EMP2 expression concomitantly resulted in decreased contraction (P = 0.002). EMP2 overexpression resulted in reduced proliferation, migration, and integrin alpha1 and alpha2 integrin expression. EMP2 overexpression was associated with a 70% increase in FAK activation (P = 0.0003) and relative resistance of gel contraction to inhibitors of FAK/Src activation.
Conclusions: ARPE-19-mediated collagen gel contraction is a multistep process that requires integrin ligation and activation of the FAK/Src complex. EMP2 positively modulates collagen gel contraction by ARPE-19 cells through increased FAK activation.
Conflict of interest statement
Disclosure: S.A. Morales, None; S. Mareninov, None; M. Wadehra, None; L. Zhang, None; L. Goodglick, None; J. Braun, None; L.K. Gordon, None
Figures
FIGURE 1
Recombinant modification of EMP2 expression. (a) Steady state protein levels of EMP2 were measured by Western blot analysis in ARPE-19 cells (control cells), ARPE-19/EMP2 cells (increased EMP2), and ARPE-19/Ribo cells (decreased EMP2). To measure the increase in EMP2 expression, various dilutions (1:10, 1:25, 1:50, and 1:100) of ARPE-19/EMP2 cell lysates were evaluated. (b) ARPE-19 cells were transiently transfected with siRNA specific for EMP2 (ARPE-19/EMP2 siRNA) or with a control scramble siRNA (ARPE-19/conrol siRNA). Experiments were performed independently at least three times, with similar results.
FIGURE 2
EMP2 modification affected collagen gel contraction. A collagen gel contraction assay was performed in ARPE-19/EMP2, ARPE-19/Ribo, and ARPE-19 cells. Cell lines with elevated and decreased EMP2 expression resulted in a 57% increase and 55% decrease in gel contraction, respectively (P = 0.002). Experiments were performed independently at least three times with similar results.
FIGURE 3
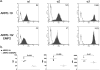
Collagen binding integrins. Cell surface expression was measured by flow cytometry with monoclonal antibodies against α1, α2, or α3 integrin. (a) Histograms of representative experiments are presented. The isotype control staining is shown as an open tracing, and the specific staining pattern is shaded. A numerical value for the mean fluorescence intensity is presented in the top right corner of each panel. (b) The surface expression of each of these integrins was evaluated in three independent experiments and the results tabulated with the mean presented. Statistical comparison of expression of each integrin in the two cell lines was performed with Student’s _t_-test (unpaired, one-tailed).
FIGURE 4
Increased EMP levels decreased proliferation and migration but had no effect on invasion or collagen production. (a) ARPE-19 and ARPE-19/EMP2 cells were treated with normal medium or 25 mg/mL collagen I and incubated for 48 hours, and proliferation was assessed by BrdU incorporation. (b) The effect of EMP2 expression on cell migration. ARPE-19 and ARPE-19/EMP2 cells were plated in a 24-well plate, and once confluent, a scratch was made and migration was measured at various time points. ARPE-19 and ARPE-19/EMP2 cells were either left untreated or were treated with 50 ng/mL PDGF, and migration was measured after 24 hours. (c) ARPE-19 and ARPE-19/EMP2 cells were seeded on an invasion chamber insert with 8-_μ_m pore size polycarbonate membrane coated with a thin layer of polymerized collagen. Either 10% FBS or 50 ng/mL PDGF was used as a chemoattractant. Invasive cells migrated through the polymerized collagen layer, clung to the bottom of the polycarbonate membrane, and were detected by staining, extraction, and measurement by a microplate reader (560 nm). (d) ARPE-19 and ARPE-19/EMP2 cells were grown in serum-free medium in the presence or absence of 10 ng/mL TGF-β for 72 hours. The media were collected and collagen production was measured by ELISA. There was no statistically significant change in collagen production in the presence or absence of TGF-β. However, TGF-β increased collagen production in each cell line compared with the untreated condition. All studies were performed at least three separate times with six wells per sample. The results were evaluated for statistical significance with a Student’s _t_-test (unpaired, one-tailed). P < 0.05 was considered to be statistically significant.
FIGURE 5
EMP2 overexpression increased FAK activation. Cell extracts (10 _μ_g protein) were fractionated by 4% to 20% SDS-PAGE gradient gel in reducing conditions, and Western immunoblots were probed with antibodies for FAK, pFAK, and _β_-actin. (a) Representative immunoblots; (b) experiments were performed independently at least three times with similar results. Band density, normalized to the _β_-actin loading control, was quantitated. Experiments were performed independently at least three times with similar results.
FIGURE 6
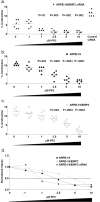
EMP2 overexpression increased resistance to PP2. Cells were pretreated for 1 hour with various concentrations of the small-molecule inhibitor PP2 (FAK/Src inhibitor), and gel contraction was assessed. The experiment was preformed at least three separate times with six replicates per sample, and a representative experiment is presented (a–c). Statistical analysis was performed with Student’s _t_-test for each concentration of inhibitor compared with the vehicle-only control. (a) ARPE-19/EMP2 siRNA cells with decreased EMP2 expression. (b) ARPE-19 cells. (c) EMP2-overexpressing ARPE-19/EMP2 cells. (d) Each point represents the average inhibition of contraction at each concentration for each cell line normalized to total contraction of the same cell line exposed to the vehicle control. Experiments were performed independently at least three times with similar results.
FIGURE 7
EMP2 overexpression increased resistance to inhibition by SU6656. Cells were pretreated for 1 hour with various concentrations of the small-molecule inhibitor SU6656 (a FAK/Src inhibitor), and gel contraction was assessed. The experiment was preformed at least three separate times with six replicates per sample, and a representative experiment is presented. Statistical analysis was performed with a Student’s _t_-test for each concentration of inhibitor compared with the vehicle-only control. (a) ARPE-19/EMP2 cells. (b) EMP2 overexpressing ARPE-19/EMP2 cells. (c) Each point represents the average inhibition of contraction at each concentration for each cell line normalized to total contraction of the same cell line exposed to the vehicle control. Experiments were performed independently at least three times with similar results.
Similar articles
- Anti-EMP2 diabody blocks epithelial membrane protein 2 (EMP2) and FAK mediated collagen gel contraction in ARPE-19 cells.
Morales SA, Telander DG, Mareninov S, Nagy A, Wadehra M, Braun J, Gordon LK. Morales SA, et al. Exp Eye Res. 2012 Sep;102:10-6. doi: 10.1016/j.exer.2012.06.002. Epub 2012 Jun 19. Exp Eye Res. 2012. PMID: 22728127 Free PMC article. - The Multifaceted Role of Epithelial Membrane Protein 2 in Cancer: from Biomarker to Therapeutic Target.
Jang JY, Park MK, Lee CH, Lee H. Jang JY, et al. Biomol Ther (Seoul). 2024 Nov 1;32(6):697-707. doi: 10.4062/biomolther.2024.168. Epub 2024 Oct 21. Biomol Ther (Seoul). 2024. PMID: 39428387 Free PMC article. Review. - Rewiring integrin-mediated signaling and cellular response with the peripheral myelin protein 22 and epithelial membrane protein 2 components of the tetraspan web.
Morales SA, Telander D, Notterpek L, Wadehra M, Braun J, Gordon LK. Morales SA, et al. Invest Ophthalmol Vis Sci. 2011 Jul 23;52(8):5465-72. doi: 10.1167/iovs.10-6139. Invest Ophthalmol Vis Sci. 2011. PMID: 21421883 Free PMC article. - Functional consequences of interactions between FAK and epithelial membrane protein 2 (EMP2).
Morales SA, Mareninov S, Coulam P, Wadehra M, Goodglick L, Braun J, Gordon LK. Morales SA, et al. Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4949-56. doi: 10.1167/iovs.08-3315. Epub 2009 Jun 3. Invest Ophthalmol Vis Sci. 2009. PMID: 19494199 Free PMC article. - Collagen gel contraction by ARPE-19 cells is mediated by a FAK-Src dependent pathway.
Morales SA, Mareninov S, Prasad P, Wadehra M, Braun J, Gordon LK. Morales SA, et al. Exp Eye Res. 2007 Dec;85(6):790-8. doi: 10.1016/j.exer.2007.08.014. Epub 2007 Aug 29. Exp Eye Res. 2007. PMID: 17915217
Cited by
- Anti-EMP2 diabody blocks epithelial membrane protein 2 (EMP2) and FAK mediated collagen gel contraction in ARPE-19 cells.
Morales SA, Telander DG, Mareninov S, Nagy A, Wadehra M, Braun J, Gordon LK. Morales SA, et al. Exp Eye Res. 2012 Sep;102:10-6. doi: 10.1016/j.exer.2012.06.002. Epub 2012 Jun 19. Exp Eye Res. 2012. PMID: 22728127 Free PMC article. - Epithelial membrane protein 2 controls VEGF expression in ARPE-19 cells.
Morales SA, Telander DG, Leon D, Forward K, Braun J, Wadehra M, Gordon LK. Morales SA, et al. Invest Ophthalmol Vis Sci. 2013 Mar 28;54(3):2367-72. doi: 10.1167/iovs.12-11013. Invest Ophthalmol Vis Sci. 2013. PMID: 23439602 Free PMC article. - The Multifaceted Role of Epithelial Membrane Protein 2 in Cancer: from Biomarker to Therapeutic Target.
Jang JY, Park MK, Lee CH, Lee H. Jang JY, et al. Biomol Ther (Seoul). 2024 Nov 1;32(6):697-707. doi: 10.4062/biomolther.2024.168. Epub 2024 Oct 21. Biomol Ther (Seoul). 2024. PMID: 39428387 Free PMC article. Review. - Epithelial Membrane Protein 2 (EMP2) Promotes VEGF-Induced Pathological Neovascularization in Murine Oxygen-Induced Retinopathy.
Sun M, Wadehra M, Casero D, Lin MC, Aguirre B, Parikh S, Matynia A, Gordon L, Chu A. Sun M, et al. Invest Ophthalmol Vis Sci. 2020 Feb 7;61(2):3. doi: 10.1167/iovs.61.2.3. Invest Ophthalmol Vis Sci. 2020. PMID: 32031575 Free PMC article. - Rewiring integrin-mediated signaling and cellular response with the peripheral myelin protein 22 and epithelial membrane protein 2 components of the tetraspan web.
Morales SA, Telander D, Notterpek L, Wadehra M, Braun J, Gordon LK. Morales SA, et al. Invest Ophthalmol Vis Sci. 2011 Jul 23;52(8):5465-72. doi: 10.1167/iovs.10-6139. Invest Ophthalmol Vis Sci. 2011. PMID: 21421883 Free PMC article.
References
- Wang CX, Wadehra M, Fisk BC, Goodglick L, Braun J. Epithelial membrane protein 2, a 4-transmembrane protein that suppresses B-cell lymphoma tumorigenicity. Blood. 2001;97(12):3890–3895. - PubMed
- Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003;74(2):106–112. - PubMed
- Wadehra M, Forbes A, Pushkarna N, et al. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. Dev Biol. 2005;287(2):336–345. - PubMed
- Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277(43):41094–41100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI52031/AI/NIAID NIH HHS/United States
- R03 HD048540/HD/NICHD NIH HHS/United States
- R01 EY019909/EY/NEI NIH HHS/United States
- T32 AI052031/AI/NIAID NIH HHS/United States
- HD48540/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous