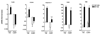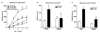Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages - PubMed (original) (raw)
Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages
Aiping Zhao et al. Gastroenterology. 2008 Jul.
Abstract
Background & aims: Enteric nematode infection induces a strong type 2 T helper cell (Th2) cytokine response characterized by increased infiltration of various immune cells, including macrophages. The role of these immune cells in host defense against nematode infection remains poorly defined. The present study investigated the role of macrophages and the arginase pathway in nematode-induced changes in intestinal smooth muscle function and worm expulsion.
Methods: Mice were infected with Nippostrongylus brasiliensis and treated with clodronate-containing liposome to deplete macrophages or given S-(2-boronoethyl)-I-cysteine in drinking water to inhibit arginase activity. Segments of intestinal smooth muscle were suspended in organ baths to determine responses to acetylcholine, 5-hydroxytryptamine, or nerve stimulation. The phenotype of macrophages was monitored by measuring mRNA expression of the specific molecular markers by real-time polymerase chain reaction or viewed by immunofluorescence staining.
Results: Infection increased the infiltration of macrophages and up-regulation alternatively activated macrophage markers by a mechanism dependent on interleukin-4 (IL-4) or interleukin-13 (IL-13) activation of signal transducer and activator of transcription 6. Elimination of alternatively activated macrophages blocked smooth muscle hypercontractility and the increased smooth muscle thickness, and impaired worm expulsion. In addition, specific inhibition of arginase activity interfered with smooth muscle contractility, but only partially affected the protective immunity of the host.
Conclusions: These data show that the phenotype of macrophages is determined by the local immune environment and that alternatively activated macrophages play a major role in the effects of Th2 cytokines, IL-4 and IL-13, on intestinal smooth muscle function.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Figure 1
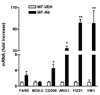
N. brasiliensis infection induced changes in the mRNA expressions of macrophage molecular markers. Mice were inoculated subcutaneously with 500 N. brasiliensis (Nb) infective third stage larvae or treated with vehicle (VEH), and studied 9 days later. Intestinal strips were taken from the mice for total RNA extraction. Real-time quantitative PCR was performed to measure the mRNA expression. The fold increases were relative to the individual vehicle groups (VEH) after normalization to 18s rRNA. * p<0.05, **p<0.01 compared with the respective WT-VEH (n≥5 for each group).
Figure 2
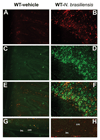
Increased infiltration and alternative activation of macrophages in the whole section (A–F) or smooth muscle layer (G, H) of small intestine from mice infected with N. brasiliensis. Mice were inoculated subcutaneously with 500 N. brasiliensis infective third stage larvae (B, D, F, H) or treated with vehicle (A, C, E, G), and studied 9 days later. Frozen tissue blocks of mid-jejunum were prepared and the sections were cut for immunofluoresence staining for anti-F4/80-Alexa647 (A, B) or anti-CD206-FITC (C, D). Fluorescent channels were photographed separately and then merged together to locate the alternatively activated macrophages in the whole section of the small intestine (E, F). For smooth muscle layer, only the merged picture is shown (G, H). All the pictures are the representatives from each group of at least 5 mice. Original magnification, x200; lm: longitudinal smooth muscle layer; cm: circular smooth muscle layer.
Figure 3
Dependence of infection-induced macrophage recruitment and activation on IL-4/IL-13 activating Stat6 and innate verse adaptive immune response. WT, SCID, IL-4−/−, IL-13−/−, or Stat6−/− mice were inoculated subcutaneously with 500 N. brasiliensis (Nb) infective third stage larvae or treated with vehicle (VEH), and studied 9 days later. One group of SCID mice was given (i.v.) exogenous IL-13 for 7 days. Intestinal strips were taken for total RNA extraction. Real-time quantitative PCR was performed to measure the mRNA expression. The fold increases were relative to the individual vehicle groups (VEH) after normalization to 18s rRNA. * p<0.05, **p<0.01 compared with the respective WT-VEH (n≥5 for each group).
Figure 4
Clodronate-liposome treatment depleted both resident and recruited macrophages in the intestine, indicated by the decreased mRNA expressions of macrophage markers in uninfected mice, or abolishing of the upregulation of the markers in _N. brasiliensis_–infected mice. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate- (Cl2MDP) or control PBS- (PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Intestinal strips were taken for total RNA extraction. Real-time quantitative PCR was performed to measure the mRNA expression. The fold increases were relative to the individual vehicle groups (VEH) after normalization to 18s rRNA. *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Figure 5
Depletion of macrophages by clodronate-liposome treatment attenuated nematode infection-induced intestinal smooth muscle hypercontractility. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate- (Cl2MDP) or control PBS- (PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Intestinal strips were taken from the mice and suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH), (C) serotonin (5-HT), or (B) for spontaneous contraction. *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Figure 6
Depletion of macrophages abolished nematode infection-induced increase in intestinal smooth muscle thickness (A) and was associated with a reduced mRNA expression of IGF-1 (B). Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate-(Cl2MDP) or control PBS- (PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Changes in smooth muscle thickness were assessed in Giemsa-stained sections (A); or whole tissue was processed for the measurement of mRNA expression of IGF-1 by real-time quantitative PCR (B). *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Figure 7
Inhibition of arginase abolished nematode infection-induced intestinal smooth muscle hypercontractility. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH), and were given 0.2% S-(2-boronoethyl)-I-cysteine (BEC) via drinking water at day 2–9 post infection for arginase inhibition in vivo. Intestinal strips were taken from the mice and suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH, 10nM-0.1mM), (C) serotonin (5-HT, 100µM), or (B) for spontaneous contraction. *p<0.05 vs the respective WT-VEH H2O; ϕp<0.05 vs the respective WT-Nb H2O (n≥5 for each group).
Figure 8
Depletion of macrophages resulted in impaired expulsion of N. brasiliensis, but did not affect N. brasiliensis infection-induced upregulation of IL-4 or IL-13. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate- (Cl2MDP) or control PBS-(PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Intestinal strips were taken for the measurement of the mRNA expression of IL-4 and IL-13 by real-time quantitative PCR (A). Separate groups of mice were infected with N. brasiliensis and treated with Cl2MDP. At the day 9 post infection, the intestine was collected for worm counting (B) and feces was collected for egg counting (C). *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Similar articles
- Critical role of IL-25 in nematode infection-induced alterations in intestinal function.
Zhao A, Urban JF Jr, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. Zhao A, et al. J Immunol. 2010 Dec 1;185(11):6921-9. doi: 10.4049/jimmunol.1000450. Epub 2010 Oct 25. J Immunol. 2010. PMID: 20974983 Free PMC article. - Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves.
Zhao A, McDermott J, Urban JF Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Zhao A, et al. J Immunol. 2003 Jul 15;171(2):948-54. doi: 10.4049/jimmunol.171.2.948. J Immunol. 2003. PMID: 12847266 - Type 3 muscarinic receptors contribute to intestinal mucosal homeostasis and clearance of Nippostrongylus brasiliensis through induction of TH2 cytokines.
McLean LP, Smith A, Cheung L, Urban JF Jr, Sun R, Grinchuk V, Desai N, Zhao A, Raufman JP, Shea-Donohue T. McLean LP, et al. Am J Physiol Gastrointest Liver Physiol. 2016 Jul 1;311(1):G130-41. doi: 10.1152/ajpgi.00461.2014. Epub 2016 May 12. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27173511 Free PMC article. - Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites.
Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF Jr. Finkelman FD, et al. Immunol Rev. 2004 Oct;201:139-55. doi: 10.1111/j.0105-2896.2004.00192.x. Immunol Rev. 2004. PMID: 15361238 Review. - Immune polarization by hookworms: taking cues from T helper type 2, type 2 innate lymphoid cells and alternatively activated macrophages.
Nair MG, Herbert DR. Nair MG, et al. Immunology. 2016 Jun;148(2):115-24. doi: 10.1111/imm.12601. Epub 2016 Mar 31. Immunology. 2016. PMID: 26928141 Free PMC article. Review.
Cited by
- IL-25 Treatment Improves Metabolic Syndrome in High-Fat Diet and Genetic Models of Obesity.
Smith AD, Fan A, Qin B, Desai N, Zhao A, Shea-Donohue T. Smith AD, et al. Diabetes Metab Syndr Obes. 2021 Dec 21;14:4875-4887. doi: 10.2147/DMSO.S335761. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34992396 Free PMC article. - Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis.
Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Pesce JT, et al. PLoS Pathog. 2009 Apr;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. Epub 2009 Apr 10. PLoS Pathog. 2009. PMID: 19360123 Free PMC article. - Myeloid cell recruitment versus local proliferation differentiates susceptibility from resistance to filarial infection.
Campbell SM, Knipper JA, Ruckerl D, Finlay CM, Logan N, Minutti CM, Mack M, Jenkins SJ, Taylor MD, Allen JE. Campbell SM, et al. Elife. 2018 Jan 4;7:e30947. doi: 10.7554/eLife.30947. Elife. 2018. PMID: 29299998 Free PMC article. - Studying the mononuclear phagocyte system in the molecular age.
Chow A, Brown BD, Merad M. Chow A, et al. Nat Rev Immunol. 2011 Oct 25;11(11):788-98. doi: 10.1038/nri3087. Nat Rev Immunol. 2011. PMID: 22025056 Review. - Trichuris muris research revisited: a journey through time.
Hurst RJ, Else KJ. Hurst RJ, et al. Parasitology. 2013 Sep;140(11):1325-39. doi: 10.1017/S0031182013001054. Parasitology. 2013. PMID: 23965819 Free PMC article. Review.
References
- Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. - PubMed
- Gordon S. ALTERNATIVE ACTIVATION OF MACROPHAGES. Nat Rev Immunol. 2003;3:23–35. - PubMed
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. - PubMed
- Raes G, De BP, Noel W, Beschin A, Brombacher F, Hassanzadeh GG. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI031678/AI/NIAID NIH HHS/United States
- R01 DK083418/DK/NIDDK NIH HHS/United States
- R01 AI049316/AI/NIAID NIH HHS/United States
- R01 AI031678-12/AI/NIAID NIH HHS/United States
- R01-AI031678/AI/NIAID NIH HHS/United States
- R01 AI049316-07/AI/NIAID NIH HHS/United States
- R01 AI031678-13/AI/NIAID NIH HHS/United States
- R01 AI049316-08/AI/NIAID NIH HHS/United States
- R01 DK049316/DK/NIDDK NIH HHS/United States
- R01-AI/DK49316/AI/NIAID NIH HHS/United States
- R01 AI031678-14/AI/NIAID NIH HHS/United States
- T32 DK067872/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials