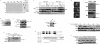ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1 - PubMed (original) (raw)
ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1
Hideki Nishitoh et al. Genes Dev. 2008.
Abstract
Mutation in Cu/Zn-superoxide dismutase (SOD1) is a cause of familial amyotrophic lateral sclerosis (ALS). Mutant SOD1 protein (SOD1(mut)) induces motor neuron death, although the molecular mechanism of SOD1(mut)-induced cell death remains controversial. Here we show that SOD1(mut) specifically interacted with Derlin-1, a component of endoplasmic reticulum (ER)-associated degradation (ERAD) machinery and triggered ER stress through dysfunction of ERAD. SOD1(mut)-induced ER stress activated the apoptosis signal-regulating kinase 1 (ASK1)-dependent cell death pathway. Perturbation of binding between SOD1(mut) and Derlin-1 by Derlin-1-derived oligopeptide suppressed SOD1(mut)-induced ER stress, ASK1 activation, and motor neuron death. Moreover, deletion of ASK1 mitigated the motor neuron loss and extended the life span of SOD1(mut) transgenic mice. These findings demonstrate that ER stress-induced ASK1 activation, which is triggered by the specific interaction of Derlin-1 with SOD1(mut), is crucial for disease progression of familial ALS.
Figures
Figure 1.
SOD1mut triggers ER stress and inhibition of ERAD. (A) NSC34 cells were lysed after infection with Ad-SOD1wt, Ad-SOD1A4V, Ad-SOD1G85R, or Ad-SOD1G93A for 48 h or treatment with 2.5 μg/mL tunicamycin (a potent inducer of ER stress) for 2 h and analyzed by immunoprecipitation-immunoblotting (IP-IB) with antibodies to IRE1α or PERK. (P-IRE1) Activated IRE1; (P-PERK) activated PERK. The presence of SOD1 in the same lysates is shown. (B) NSC34 cells were transfected with SOD1wt or SOD1mut for 48 h. Expression of BiP and CHOP was examined by RT–PCR. (C) NSC34 cells were transfected with NHK and SOD1wt or SOD1mut. Cells were pulse-labeled with [35S] methionine and cysteine and chased for the indicated time periods. Cell lysates were immunoprecipitated with antibody to α1AT. (NHK + CHO) Glycosylated NHK; (NHK − CHO) deglycosylated NHK. The relative radioactivities in NHK at different times of chase were calculated and are shown as fold decreases relative to the intensity observed at 0 h chase. (D) NSC34 cells were transfected with VenusU and SOD1wt or SOD1mut. Cells were pulse-labeled with [35S] methionine and cysteine and chased for the indicated time periods. Cell lysates were immunoprecipitated with antibody to GFP. The relative radioactivities in VenusU at different times of chase were calculated and are shown as fold decreases relative to the intensity observed at 0 h chase.
Figure 2.
SOD1mut interacts with Derlin-1. (A) 35S-labeled p97, Ufd1, Npl4, VIMP, or Derlin-1 proteins were incubated with recombinant His-SOD1wt or -SOD1G93A proteins and immobilized on Ni-NTA beads (top left). The bottom part of the SDS-PAGE gel was stained with Coomassie brilliant blue dye (CBB). Amounts of incubated 35S-labeled proteins are shown (input). (B) Lysates from HEK293 cells, transfected at the indicated combinations, were analyzed by IP-IB. (C) NSC34 cells were lysed after infection with Ad-Flag-SOD1wt, Ad-Flag-SOD1A4V, Ad-Flag-SOD1G85R, or Ad-Flag-SOD1G93A for 48 h and analyzed by IP-IB. (D) Extracts from spinal cords of mice were immunoprecipitated with an antibody to SOD1 (IP: SOD1) or control nonimmune antibody (IP: C) and analyzed by IB with antibodies to Derlin-1 and SOD1. The presence of Derlin-1 in the same lysates is shown. (E) Extracts from tissues of SOD1wt mice and SOD1G93A mice were analyzed by IB with antibodies to Derlin-1, MAP2, or p38. Interaction between SOD1mut and Derlin-1 in tissues of SOD1G93A mice was analyzed by IP-IB. (F) Spinal cord of SOD1G93A mouse was homogenized and the microsomes were isolated from the post-nuclear and post-mitochondria homogenate. For alkaline extraction, the microsomes were incubated in 100 mM Na2CO3 (pH 7.5 or pH 10.9). After the incubation, the microsomes were pelleted and analyzed by IB with antibodies to Derlin-1, SOD1, and p97. Derlin-1 and p97 were used as controls for ER membrane-anchored protein and peripheral protein, respectively. (G) Schematic representation of various mutant forms of Derlin-1. Transmembrane domains of Derlin-1 are shown by black boxes. Identical residues of C-terminal fragment of Derlin family proteins are shaded. (H) HEK293 cells were transfected with various mutant forms of Derlin-1 and stained with antibody to HA. (I–K) Lysates from HEK293 cells, transfected at the indicated combinations, were analyzed by IP-IB.
Figure 3.
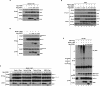
SOD1mut attenuates the retro-translocation of ERAD substrate on the components of ERAD. (A) Lysates from HEK293 cells, transfected with Derlin-1-HA, Myc-VIMP, and Flag-SOD1 at the indicated combinations, were analyzed by IP-IB. The presence of Derlin-1-HA and Flag-SOD1 in the same lysates is shown. (B) Lysates from HEK293 cells, transfected at the indicated combinations, were analyzed by IP-IB. The presence of p97 and Myc-SOD1 in the same lysates is shown. (C) Lysates from HEK293 cells, transfected at the indicated combinations, were analyzed by IP-IB. The presence of Derlin-1-HA and Myc-SOD1 in the same lysates is shown. (D) Lysates from HEK293 cells, transfected with NHK, Derlin-1-HA, Myc-VIMP, and Flag-SOD1 at the indicated combinations, were analyzed by IP-IB. The presence of Derlin-1-HA, Myc-VIMP, and Flag-SOD1 in the same lysates is shown. (E) HEK293 cells were transfected with NHK-Flag, Derlin-1-HA, Myc-SOD1, and HA-Ub at the indicated combinations and incubated with 0.25 μM MG132 for 18 h. NHK was immunoprecipitated with antibody to Flag. After incubation with the denaturing buffer containing 1% SDS, NHK was reimmunoprecipitated with antibody to Flag. Samples were immunoblotted with antibodies to HA and Flag. The presence of Derlin-1-HA and Myc-SOD1 in the same lysates is shown. Asterisks denote nonspecific bands and IgG.
Figure 4.
SOD1mut activates the IRE1–TRAF2–ASK1 pathway. (A) NSC34 cells were infected with Ad-SOD1wt, Ad-SOD1A4V, Ad-SOD1G85R, or Ad-SOD1G93A for 48 h. ASK1 activity was measured by in vitro kinase assay (IVK). (GST-SEK1KNP) Phosphorylated GST-SEK1KN. Interaction between TRAF2 and ASK1 was analyzed by IP with antibody to TRAF2 and IB with antibody to ASK1. The presence of ASK1, TRAF2, and SOD1 in the same lysates is shown. (Lane 1) Kinase activity relative to the amount of ASK1 protein is shown as fold increase compared with control cells. (B) NSC34 cells were infected with adenoviruses and lentivirus at the indicated combinations. SOD1mut-dependent IRE1–TRAF2–ASK1 complex formation were analyzed by IP-IB. (C) NSC34 cells, transfected with siRNA against Derlin-1 (si1) or nonspecific sequence (Control), were infected with Ad-SOD1wt or Ad-SOD1G93A for 48 h or treatment with 10 μM Thapsigargin for 2 h. Activation of IRE1 and ASK1 was analyzed by IP-IB with antibodies to IRE1α and by IVK using GST-SEK1KN as a substrate, respectively. The presence of ASK1, Derlin-1, Derlin-2, SOD1, and p38 in the same lysates is shown. (Lanes 1,5) Kinase activity relative to the amount of ASK1 protein is shown as fold increase compared with control cells. (D,E) NSC34 cells, transfected with siRNA against Derlin-1 (si1), Derlin-1 (si2), or nonspecific sequence (Control), were infected with Ad-SOD1wt, Ad-SOD1A4V, Ad-SOD1G85R, or Ad-SOD1G93A for 48 h or treatment with 2 μM thapsigargin for 2 h. Xbp-1 mRNA splicing was determined by RT–PCR. Interaction between TRAF2 and ASK1 was analyzed by IP with antibody to TRAF2 and IB with antibody to ASK1. The presence of TRAF2, ASK1, Derlin-1, and Flag-SOD1 in the same lysates is shown.
Figure 5.
Impairment of SOD1mut–Derlin-1 interaction and deletion of ASK1 mitigate SOD1mut-induced motor neurotoxicity. (A) Lysates from HEK293 cells, transfected at the indicated combinations, were analyzed by IP-IB. (B) NSC34 cells were infected with Ad-SOD1wt, Ad-SOD1G93A, Len-Venus-Derlin-1(CT4)-HA, and Len-Venus-HA at the indicated combinations for 48 h. Activation of IRE1 and PERK was examined as described in Figure 1A. Activation of ASK1 was analyzed by IVK using GST-MKK6KN as substrate. (ASK1P) Autophosphorylated ASK1. (GST-MKK6KNP) Phosphorylated GST-MKK6KN. Asterisk denotes nonspecific bands. (C) Wild-type mice spinal cord cultures were infected with lentivirus at the indicated combinations for 72 h. Cultures were fixed and doubly stained with antibodies to nonphosphorylated neurofilament (SMI32) (green) and GFAP (red). (D) ASK1−/− mice spinal cord cultures were infected as indicated and stained with SMI32 antibody. (E) The percentage of total cell count of SMI32 antibody-positive cells is shown compared with control culture (wild type; n = 3); (ASK1−/−; n = 5). Values are means ± SE of independent experiments. (*) P < 0.05; (**) P < 0.01; significance calculated by Student’s _t_-test. (#) P < 0.05 significance calculated by ANOVA. (F) The percentage of total cell count of anti-GFAP antibody-positive cells derived from wild-type mice is shown compared with control culture. Values are means ± SE of three independent experiments. (G) The onset of disease was determined by motor function deficit seen in rota-rod performance in SOD1G93A mice in the presence or absence (ASK1−/−) of ASK1. The cumulative probability of onset of rota-rod deficit was not significantly changed in SOD1G93A/ASK1−/− mice (n = 10; solid line) compared with SOD1G93A mice (n = 10; dotted line). Probabilities of survival reveal prolongation of life span of SOD1G93A/ASK1−/− mice (n = 20; solid line) compared with SOD1G93A mice (n = 20; dotted line). The data were analyzed by the Kaplan-Meier life test and by long-rank test. (H) Cresyl violet (Nissl)-stained paraffin sections of ventral horn from lumbar (level L3) spinal cords at end stage (age 34 wk) are shown. (I) Stereological analysis of motor neuron death. Numbers of motor neurons were determined by counting the large Nissl-positive neurons in the ventral horn. Five mice of each group were used for analysis. Thirty-week-old and 34-wk-old mice were sacrificed, and sections of lumbar spinal cord at levels L3, L4, and L5 were counted for each mouse. Values are means ± SE. (*) P < 0.05; (**) P < 0.01; significance calculated by Student’s _t_-test.
Figure 6.
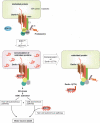
Schematic representation of the mechanism of SOD1mut-induced motor neuron death through ER stress. See the text for details.
Similar articles
- Superoxide dismutase 1 mutants related to amyotrophic lateral sclerosis induce endoplasmic stress in neuro2a cells.
Oh YK, Shin KS, Yuan J, Kang SJ. Oh YK, et al. J Neurochem. 2008 Feb;104(4):993-1005. doi: 10.1111/j.1471-4159.2007.05053.x. J Neurochem. 2008. PMID: 18233996 - A novel monoclonal antibody reveals a conformational alteration shared by amyotrophic lateral sclerosis-linked SOD1 mutants.
Fujisawa T, Homma K, Yamaguchi N, Kadowaki H, Tsuburaya N, Naguro I, Matsuzawa A, Takeda K, Takahashi Y, Goto J, Tsuji S, Nishitoh H, Ichijo H. Fujisawa T, et al. Ann Neurol. 2012 Nov;72(5):739-49. doi: 10.1002/ana.23668. Ann Neurol. 2012. PMID: 23280792 - A systematic immunoprecipitation approach reinforces the concept of common conformational alterations in amyotrophic lateral sclerosis-linked SOD1 mutants.
Fujisawa T, Yamaguchi N, Kadowaki H, Tsukamoto Y, Tsuburaya N, Tsubota A, Takahashi H, Naguro I, Takahashi Y, Goto J, Tsuji S, Nishitoh H, Homma K, Ichijo H. Fujisawa T, et al. Neurobiol Dis. 2015 Oct;82:478-486. doi: 10.1016/j.nbd.2015.08.010. Epub 2015 Aug 18. Neurobiol Dis. 2015. PMID: 26297318 - [Molecular mechanisms of ALS-linked mutant SOD1-induced motor neuron death].
Nishitoh H, Ichijo H. Nishitoh H, et al. Tanpakushitsu Kakusan Koso. 2009 Mar;54(3):237-44. Tanpakushitsu Kakusan Koso. 2009. PMID: 19288861 Review. Japanese. No abstract available. - Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.
Shibata N. Shibata N. Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review.
Cited by
- The complex molecular biology of amyotrophic lateral sclerosis (ALS).
Redler RL, Dokholyan NV. Redler RL, et al. Prog Mol Biol Transl Sci. 2012;107:215-62. doi: 10.1016/B978-0-12-385883-2.00002-3. Prog Mol Biol Transl Sci. 2012. PMID: 22482452 Free PMC article. Review. - A Crucial Role for the Protein Quality Control System in Motor Neuron Diseases.
Cristofani R, Crippa V, Cicardi ME, Tedesco B, Ferrari V, Chierichetti M, Casarotto E, Piccolella M, Messi E, Galbiati M, Rusmini P, Poletti A. Cristofani R, et al. Front Aging Neurosci. 2020 Jul 21;12:191. doi: 10.3389/fnagi.2020.00191. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32792938 Free PMC article. - Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues.
Peters JC, Bhattacharya S, Clark AF, Zode GS. Peters JC, et al. Invest Ophthalmol Vis Sci. 2015 Jun;56(6):3860-8. doi: 10.1167/iovs.14-16220. Invest Ophthalmol Vis Sci. 2015. PMID: 26066753 Free PMC article. - Breaking barriers with tofersen: Enhancing therapeutic opportunities in amyotrophic lateral sclerosis.
Saini A, Chawla PA. Saini A, et al. Eur J Neurol. 2024 Feb;31(2):e16140. doi: 10.1111/ene.16140. Epub 2023 Nov 17. Eur J Neurol. 2024. PMID: 37975798 Free PMC article. Review. - Inhibition of Cytohesins Protects against Genetic Models of Motor Neuron Disease.
Zhai J, Zhang L, Mojsilovic-Petrovic J, Jian X, Thomas J, Homma K, Schmitz A, Famulok M, Ichijo H, Argon Y, Randazzo PA, Kalb RG. Zhai J, et al. J Neurosci. 2015 Jun 17;35(24):9088-105. doi: 10.1523/JNEUROSCI.5032-13.2015. J Neurosci. 2015. PMID: 26085633 Free PMC article.
References
- Atkin J.D., Farg M.A., Turner B.J., Tomas D., Lysaght J.A., Nunan J., Rembach A., Nagley P., Beart P.M., Cheema S.S., et al. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 2006;281:30152–30165. - PubMed
- Boillee S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G., Kollias G., Cleveland D.W. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
- Bowling A.C., Schulz J.B., Brown R.H., Beal M.F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 1993;61:2322–2325. - PubMed
- Clement A.M., Nguyen M.D., Roberts E.A., Garcia M.L., Boillee S., Rule M., McMahon A.P., Doucette W., Siwek D., Ferrante R.J., et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous