Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants - PubMed (original) (raw)
Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants
Sandra Weller et al. J Exp Med. 2008.
Abstract
T cell-dependent immune responses develop soon after birth, whereas it takes 2 yr for humans to develop T cell-independent responses. We used this dissociation to analyze the repertoire diversification of IgM(+)IgD(+)CD27(+) B cells (also known as "IgM memory" B cells), comparing these cells with switched B cells in children <2 yr of age, with the aim of determining whether these two subsets are developmentally related. We show that the repertoire of IgM(+)IgD(+)CD27(+) B cells in the spleen and blood displays no sign of antigen-driven activation and expansion on H-CDR3 spectratyping, despite the many antigenic challenges provided by childhood vaccinations. This repertoire differed markedly from those of switched B cells and splenic germinal center B cells, even at the early stage of differentiation associated with mu heavy chain expression. These data provide evidence for the developmental diversification of IgM(+)IgD(+)CD27(+) B cells, at least in very young children, outside of T cell-dependent and -independent immune responses.
Figures
Figure 1.
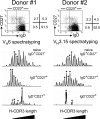
H-CDR3 spectratypes of the VH6 or VH3.15 transcripts expressed by the blood B cell subsets of two 11-mo-old children. Naive IgD+CD27−, IgD+CD27+, and switched IgD−CD27+ B cells were sorted from the blood samples of donors 1 and 2. Total RNA from each cell fraction was reverse transcribed and VH6 or VH3.15 μ or γ transcripts were amplified by PCR, using a seminested strategy (see Materials and methods). The PCR products were labeled by a run-off reaction with specific fluorescent VH-FR3 primers, and subjected to electrophoresis on an automated sequencer. The resulting size distribution of the peaks directly reflects the size distribution of H-CDR3 for the given transcripts. Peaks identified by an asterisk were further sequenced to evaluate intrapeak clonal diversity.
Figure 2.
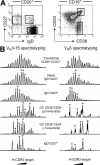
H-CDR3 spectratypes of the VH3.15 and VH6 transcripts expressed by the splenic B cell subsets of an 8-mo-old child. (A) Two different marker combinations were used to isolate splenic GC (CD19+CD24−CD38+) and transitional B cells (CD19+CD38++CD24++) or naive IgD+CD27+, IgD+CD27+ and switched IgD−CD27+ B cells. (B) Spectratyping was performed on VH3.15 and VH6 μ and/or γ transcripts from each cell fraction, as described in the legend of Fig. 1. Peaks identified by an asterisk were further sequenced to evaluate intrapeak clonal diversity.
Figure 3.
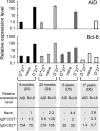
Detectable AID expression in splenic IgD+CD27+ cells from very young children. Relative AID and Bcl-6 expression levels were determined for various splenic B cell subsets from different donors. AID and B_cl-6_ sequences were amplified by real-time quantitative PCR from cDNA from IgD+CD27+, IgD+CD27− (naive), and IgD−CD27+ cells purified by two consecutive cell sortings. The results shown were obtained from two independent PCRs (except for D6), each performed in triplicate. The relative expression of AID or Bcl-6 in each subset was calculated by the comparative method, normalizing to 1 the expression of AID in the IgD+CD27+ fraction and of Bcl-6 in the naive subset of the 8-mo-old donor (D3). PCRs with a threshold cycle (Ct) >35 were considered NS.
Figure 4.
IgD+CD27+CD1chigh cells are already present in the SMZ of an 8-mo-old child. Serial splenic cryosections were double-labeled either with anti-IgD (green) and anti-CD27 (red) antibodies (A–D) or with anti-IgD (green) and anti-CD1c (red) antibodies (E–H), and then examined under a confocal microscope. CD27low cells (B and D) were present in the MZ corresponding to the outer zone of the IgD-positive ring surrounding the GC (A and C). Boxes with dotted lines in A and B indicate the zone magnified in C and D. A higher level of CD1c expression was observed in the MZ (F), resulting in a yellow appearance in the merged images (G and H), caused by the coexpression of IgD and CD1c at similar intensities. H shows higher magnification of the zone delimited by the box with dotted lines in G. Co, corona. Bars, 50 μm.
Comment in
- Sheepish B cells: evidence for antigen-independent antibody diversification in humans and mice.
Tarlinton D. Tarlinton D. J Exp Med. 2008 Jun 9;205(6):1251-4. doi: 10.1084/jem.20081057. Epub 2008 Jun 2. J Exp Med. 2008. PMID: 18519651 Free PMC article.
Similar articles
- Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation.
Seifert M, Küppers R. Seifert M, et al. J Exp Med. 2009 Nov 23;206(12):2659-69. doi: 10.1084/jem.20091087. Epub 2009 Nov 16. J Exp Med. 2009. PMID: 19917772 Free PMC article. - High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations.
Wu YC, Kipling D, Leong HS, Martin V, Ademokun AA, Dunn-Walters DK. Wu YC, et al. Blood. 2010 Aug 19;116(7):1070-8. doi: 10.1182/blood-2010-03-275859. Epub 2010 May 10. Blood. 2010. PMID: 20457872 Free PMC article. - [Molecules involved in characteristics of naive/memory B cells].
Agematsu K. Agematsu K. Nihon Rinsho Meneki Gakkai Kaishi. 2004 Oct;27(5):309-14. doi: 10.2177/jsci.27.309. Nihon Rinsho Meneki Gakkai Kaishi. 2004. PMID: 15559319 Review. Japanese. - Memory B cells and CD27.
Agematsu K. Agematsu K. Histol Histopathol. 2000 Apr;15(2):573-6. doi: 10.14670/HH-15.573. Histol Histopathol. 2000. PMID: 10809378 Review.
Cited by
- Changes in B Cell Populations and Merozoite Surface Protein-1-Specific Memory B Cell Responses after Prolonged Absence of Detectable P. falciparum Infection.
Ayieko C, Maue AC, Jura WG, Noland GS, Ayodo G, Rochford R, John CC. Ayieko C, et al. PLoS One. 2013 Jun 27;8(6):e67230. doi: 10.1371/journal.pone.0067230. Print 2013. PLoS One. 2013. PMID: 23826242 Free PMC article. - IgM memory B cells: a mouse/human paradox.
Reynaud CA, Descatoire M, Dogan I, Huetz F, Weller S, Weill JC. Reynaud CA, et al. Cell Mol Life Sci. 2012 May;69(10):1625-34. doi: 10.1007/s00018-012-0971-z. Epub 2012 Apr 6. Cell Mol Life Sci. 2012. PMID: 22481437 Free PMC article. Review. - Immunophenotyping of putative human B1 B cells in healthy controls and common variable immunodeficiency (CVID) patients.
Suchanek O, Sadler R, Bateman EA, Patel SY, Ferry BL. Suchanek O, et al. Clin Exp Immunol. 2012 Dec;170(3):333-41. doi: 10.1111/j.1365-2249.2012.04656.x. Clin Exp Immunol. 2012. PMID: 23121674 Free PMC article. - Suppression of circulating IgD+CD27+ memory B cells in infants living in a malaria-endemic region of Kenya.
Asito AS, Piriou E, Jura WG, Ouma C, Odada PS, Ogola S, Fiore N, Rochford R. Asito AS, et al. Malar J. 2011 Dec 13;10:362. doi: 10.1186/1475-2875-10-362. Malar J. 2011. PMID: 22166136 Free PMC article. - CD20 deficiency in humans results in impaired T cell-independent antibody responses.
Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA. Kuijpers TW, et al. J Clin Invest. 2010 Jan;120(1):214-22. doi: 10.1172/JCI40231. Epub 2009 Dec 21. J Clin Invest. 2010. PMID: 20038800 Free PMC article.
References
- Klein, U., R. Kuppers, and K. Rajewsky. 1997. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 89:1288–1298. - PubMed
- Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. - PMC - PubMed
- Carsetti, R., M.M. Rosado, and H. Wardmann. 2004. Peripheral development of B cells in mouse and man. Immunol. Rev. 197:179–191. - PubMed
- Cuss, A.K., D.T. Avery, J.L. Cannons, L.J. Yu, K.E. Nichols, P.J. Shaw, and S.G. Tangye. 2006. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J. Immunol. 176:1506–1516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials