Birth and rapid subcellular adaptation of a hominoid-specific CDC14 protein - PubMed (original) (raw)
Birth and rapid subcellular adaptation of a hominoid-specific CDC14 protein
Lia Rosso et al. PLoS Biol. 2008.
Abstract
Gene duplication was prevalent during hominoid evolution, yet little is known about the functional fate of new ape gene copies. We characterized the CDC14B cell cycle gene and the functional evolution of its hominoid-specific daughter gene, CDC14Bretro. We found that CDC14B encodes four different splice isoforms that show different subcellular localizations (nucleus or microtubule-associated) and functional properties. A microtubular CDC14B variant spawned CDC14Bretro through retroposition in the hominoid ancestor 18-25 million years ago (Mya). CDC14Bretro evolved brain-/testis-specific expression after the duplication event and experienced a short period of intense positive selection in the African ape ancestor 7-12 Mya. Using resurrected ancestral protein variants, we demonstrate that by virtue of amino acid substitutions in distinct protein regions during this time, the subcellular localization of CDC14Bretro progressively shifted from the association with microtubules (stabilizing them) to an association with the endoplasmic reticulum. CDC14Bretro evolution represents a paradigm example of rapid, selectively driven subcellular relocalization, thus revealing a novel mode for the emergence of new gene function.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. Splice Variants of the CDC14B Parental Gene and Subcellular Localization of Encoded Proteins
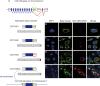
(A) Exon and protein structures of CDC14B splice variants. Color code of coding exons (exons not drawn to scale): exons shared by all variants are colored in dark blue; exons colored in yellow, light blue, green, and red are specific to transcripts CDC14B1, CDC14B2, CDC14B3, and CDC14Bpar, respectively, as indicated by the schematic representation of splicing patterns. The basic protein structure and sequence encoded by the different splice variants is indicated (only the protein sequence that differs between the splice variants is color-coded to indicate the relationship with the alternative splicing patterns of the coding exons). The retroduplication event of the CDC14Bpar transcript that gave rise to the CDC14B retrogene (CDC14Bretro) is illustrated. (B–E) COS7-cells (from African green monkeys—no endogenous CDC14Bretro gene) transfected with _CDC14B_-DsRed fusion constructs (i.e., CDC14B signals in red) are shown. Microtubules were stained using an anti-beta tubulin antibody (green) and the nucleus was stained using DAPI (blue). Merged images: CDC14B1 (nucleoli) and −2 (nuclear speckles) localize to the nucleus (violet) and CDC14B3 and CDC14Bpar co-localize with microtubules (yellow). We obtained very similar results when using HeLa or LN229 (glioblastoma) cell lines (both carrying an endogenous CDC14Bretro gene, Figure S1). Thus, there appear to be no cell line–specific effects with respect to the observed subcellular localization patterns. Scale bars (white) = 10 μm.
Figure 2. Spatial Expression Patterns of the CDC14B Parental and Retrogene Variants
Shaded areas indicate expression in a given tissue. “ND” indicates that expression was not determined. Note that weak amplification products of human CDC14Bretro were obtained for the following tissues in some of the cDNA panels tested: kidney, lung, ovary, thymus, and thyroid. This suggests that it may be expressed at low levels in these (and perhaps other) tissues in some individuals.
Figure 3. Coding Sequence Evolution of CDC14Bretro/CDC14Bpar and Subcellular Localization of Encoded Proteins
(A) Phylogenetic tree based on the CDC14B coding sequences (mouse CDC14Bpar sequence is used as an outgroup, see Materials and Methods for details). Approximate divergence times in millions of years (Mya) are shown (estimates based on [15]). Maximum likelihood d N/d S values and the estimated number of nonsynonymous over synonymous substitutions (in parentheses) for each branch are indicated (estimated numbers of substitutions are rounded to the nearest integer). Internal nodes: Duplication event (node D), Before positive selection (node B, sequence from last common great ape ancestor), and After positive selection (node A, last common African ape ancestor). The branch showing positive selection is highlighted in red. Abbreviations: human CDC14Bretro, HuRetro; chimpanzee CDC14Bretro, ChRetro; gorilla CDC14Bretro, GoRetro; orangutan CDC14Bretro, OrRetro; gibbon CDC14Bretro, GiRetro; human CDC14Bpar, HuPar. (B) CDC14Bretro from node A in interphase. COS7 cells transfected with a _CDC14Bretro_-GFP fusion construct (i.e., CDC14B signals in green) are shown. Microtubules are stained in red (anti-beta tubulin antibody). Nuclear DNA was stained with DAPI (blue). (C) Human CDC14Bretro in interphase. (D) Human CDC14Bretro during mitosis. (E) Human CDC14Bretro during cytokinesis. (F) CDC14Bretro from node B in interphase. (G) Human CDC14Bpar in interphase. (H) CDC14Bpar during mitosis. (I) CDC14Bpar during cytokinesis. Only merged images are shown; unmerged images are shown in Figure S3. Similar results were obtained when using either human HeLa or LN229 cell lines (Figure S4). Scale bars = 10 μm.
Figure 4. Alignment of Extant and Reconstructed CDC14Bretro and CDC14Bpar sequences
N and C termini are highlighted in light and dark blue, respectively. The 12 sites where amino acid changes arose in the common African ape ancestor are highlighted in orange. The amino acid motif KKIR, previously described to be crucial for nuclear localization of the parental variants CDC14B1 and CDC14B2, is highlighted in green. Sites crucial for the phosphatase function of CDC14B [17,35] are highlighted in dark red. The sequence N terminal of the new initial methionine in humans and chimpanzees is marked in gray, as this part of the sequence is likely not translated in these species. The same abbreviations as in the legend of Figure 3 were used to designate CDC14B sequences from the different species.
Figure 5. Subcellular Localization of CDC14Bretro from Humans and Node A
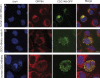
(A) Human CDC14Bretro in interphase. COS7 cells transfected with two different _CDC14Bretro_-GFP fusion constructs (green) and labeled with an anti-GRP94 antibody (red, a marker of the endoplasmic reticulum) and with DAPI (blue). (B) CDC14Bretro from node A (see Figure 3) in interphase. (C) Human CDC14Bretro during mitosis. Similar results were obtained when using either human HeLa or LN229 cell lines (Figure S8). We note that in addition to the ER, CDC14Bretro from African apes localizes to vesicles that show no co-staining with any of the cellular markers tested (see Materials and Methods) more than 24 h after transfection. Scale bars = 10 μm.
Figure 6. Subcellular Localization of Hybrid Constructs BAB and ABA
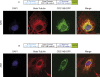
COS7 cells transfected with hybrid fusion construct BAB and ABA (i.e., CDC14B signals in green). Microtubules stained in red (anti-beta tubulin antibody). Nuclear DNA was stained with DAPI (blue). Scale bars = 10 μm.
Figure 7. Microtubule Stabilization Capacity of CDC14Bretro and Parental CDC14B Variants
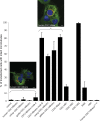
Cells transfected with CDC14Bretro/CDC14B were treated with the depolymerizing agent nocodazole (see Materials and Methods for details). All experiments were repeated five times. Representative images of COS7 cells treated with nocodazole 24 h after transfection with human CDC14Bretro and human CDC14Bpar (fused to DsRed), in which microtubules (labeled in green) are stabilized (right image, yellow/green filaments; yellow indicates CDC14Bpar-microtubule co-localization) or degraded (left image) are shown. Statistical analysis reveals significant stabilization differences between the different CDC14B proteins (one-way ANOVA, p < 10−4). Relevant pairwise comparisons of proteins that show significant stabilization differences (p < 0.01, Tukey's Post Hoc test): (i) CDC14Bretro from humans, chimpanzee, gorilla, node A versus CDC14Bretro from orangutan, gibbon, node B, CDC14Bpar, and CDC14B3; (ii) CDC14B1 and CDC14B2 versus CDC14Bpar/CDC14B3; (iii) node B versus BAB/ABA hybrids; (iv) BAB versus ABA; (v) CDC14Bretro from orangutan and gibbon versus CDC14Bretro from node B and CDC14Bpar. Scale bars = 10 μm.
Similar articles
- Mitochondrial targeting adaptation of the hominoid-specific glutamate dehydrogenase driven by positive Darwinian selection.
Rosso L, Marques AC, Reichert AS, Kaessmann H. Rosso L, et al. PLoS Genet. 2008 Aug 8;4(8):e1000150. doi: 10.1371/journal.pgen.1000150. PLoS Genet. 2008. PMID: 18688271 Free PMC article. - The nucleolar phosphatase Cdc14B is dispensable for chromosome segregation and mitotic exit in human cells.
Berdougo E, Nachury MV, Jackson PK, Jallepalli PV. Berdougo E, et al. Cell Cycle. 2008 May 1;7(9):1184-90. doi: 10.4161/cc.7.9.5792. Epub 2008 Feb 13. Cell Cycle. 2008. PMID: 18418058 - Functional diversification of duplicate genes through subcellular adaptation of encoded proteins.
Marques AC, Vinckenbosch N, Brawand D, Kaessmann H. Marques AC, et al. Genome Biol. 2008;9(3):R54. doi: 10.1186/gb-2008-9-3-r54. Epub 2008 Mar 12. Genome Biol. 2008. PMID: 18336717 Free PMC article. - The dual-specificity phosphatase CDC14B bundles and stabilizes microtubules.
Cho HP, Liu Y, Gomez M, Dunlap J, Tyers M, Wang Y. Cho HP, et al. Mol Cell Biol. 2005 Jun;25(11):4541-51. doi: 10.1128/MCB.25.11.4541-4551.2005. Mol Cell Biol. 2005. PMID: 15899858 Free PMC article. - Birth and adaptive evolution of a hominoid gene that supports high neurotransmitter flux.
Burki F, Kaessmann H. Burki F, et al. Nat Genet. 2004 Oct;36(10):1061-3. doi: 10.1038/ng1431. Epub 2004 Sep 19. Nat Genet. 2004. PMID: 15378063
Cited by
- Comparative study of human mitochondrial proteome reveals extensive protein subcellular relocalization after gene duplications.
Wang X, Huang Y, Lavrov DV, Gu X. Wang X, et al. BMC Evol Biol. 2009 Nov 30;9:275. doi: 10.1186/1471-2148-9-275. BMC Evol Biol. 2009. PMID: 19948060 Free PMC article. - Human Cdc14B promotes progression through mitosis by dephosphorylating Cdc25 and regulating Cdk1/cyclin B activity.
Tumurbaatar I, Cizmecioglu O, Hoffmann I, Grummt I, Voit R. Tumurbaatar I, et al. PLoS One. 2011 Feb 17;6(2):e14711. doi: 10.1371/journal.pone.0014711. PLoS One. 2011. PMID: 21379580 Free PMC article. - Retrogenes in rice (Oryza sativa L. ssp. japonica) exhibit correlated expression with their source genes.
Sakai H, Mizuno H, Kawahara Y, Wakimoto H, Ikawa H, Kawahigashi H, Kanamori H, Matsumoto T, Itoh T, Gaut BS. Sakai H, et al. Genome Biol Evol. 2011;3:1357-68. doi: 10.1093/gbe/evr111. Epub 2011 Oct 31. Genome Biol Evol. 2011. PMID: 22042334 Free PMC article. - The DNA damage effector Chk1 kinase regulates Cdc14B nucleolar shuttling during cell cycle progression.
Peddibhotla S, Wei Z, Papineni R, Lam MH, Rosen JM, Zhang P. Peddibhotla S, et al. Cell Cycle. 2011 Feb 15;10(4):671-9. doi: 10.4161/cc.10.4.14901. Epub 2011 Feb 15. Cell Cycle. 2011. PMID: 21301228 Free PMC article. - RNA-Mediated Gene Duplication and Retroposons: Retrogenes, LINEs, SINEs, and Sequence Specificity.
Ohshima K. Ohshima K. Int J Evol Biol. 2013;2013:424726. doi: 10.1155/2013/424726. Epub 2013 Aug 1. Int J Evol Biol. 2013. PMID: 23984183 Free PMC article.
References
- Ohno S. Evolution by gene duplication. Berlin: Springer Verlag; 1970.
- Samonte RV, Eichler EE. Segmental duplications and the evolution of the primate genome. Nature Rev Genet. 2002;3:65–72. - PubMed
- Brosius J. Retroposons–seeds of evolution. Science. 1991;251:753. - PubMed
- Long M, Betran E, Thornton K, Wang W. The origin of new genes: glimpses from the young and old. Nature Rev Genet. 2003;4:865–875. - PubMed
- Bailey JA, Eichler EE. Primate segmental duplications: crucibles of evolution, diversity and disease. Nature Rev Genet. 2006;7:552–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous