Positive feedback sharpens the anaphase switch - PubMed (original) (raw)
. 2008 Jul 17;454(7202):353-7.
doi: 10.1038/nature07050. Epub 2008 Jun 15.
Affiliations
- PMID: 18552837
- PMCID: PMC2636747
- DOI: 10.1038/nature07050
Positive feedback sharpens the anaphase switch
Liam J Holt et al. Nature. 2008.
Abstract
At the onset of anaphase, sister-chromatid cohesion is dissolved abruptly and irreversibly, ensuring that all chromosome pairs disjoin almost simultaneously. The regulatory mechanisms that generate this switch-like behaviour are unclear. Anaphase is initiated when a ubiquitin ligase, the anaphase-promoting complex (APC), triggers the destruction of securin, thereby allowing separase, a protease, to disrupt sister-chromatid cohesion. Here we demonstrate that the cyclin-dependent kinase 1 (Cdk1)-dependent phosphorylation of securin near its destruction-box motif inhibits securin ubiquitination by the APC. The phosphatase Cdc14 reverses securin phosphorylation, thereby increasing the rate of securin ubiquitination. Because separase is known to activate Cdc14 (refs 5 and 6), our results support the existence of a positive feedback loop that increases the abruptness of anaphase. Consistent with this model, we show that mutations that disrupt securin phosphoregulation decrease the synchrony of chromosome segregation. Our results also suggest that coupling securin degradation with changes in Cdk1 and Cdc14 activities helps coordinate the initiation of sister-chromatid separation with changes in spindle dynamics.
Figures
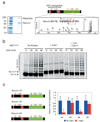
Figure 1. Cdk1 and Cdc14 control the phosphorylation state of securin near its destruction-box and modulate the rate of securin ubiquitination
a, Securin was purified from a benomyl-arrested strain carrying SECURIN-3FLAG-6HIS (left panel), and MALDI-MS/MS analysis resulted in the detection of ten phosphopeptides (Suppl. Fig. 1), including four peptides phosphorylated at Cdk1 consensus sites (red). One of these sites lies next to the securin destruction-box motif (the MS3 spectrum of the phosphopeptide is shown to the right, with the phosphopeptide sequence most consistent with the data). b, 35S-methionine-labeled securin was produced by translation in vitro and incubated with purified APCCdc20 and other ubiquitination components. Prior to the reactions, as indicated, some samples were incubated with either purified Clb2-Cdk1 or with Clb2-Cdk1 and then Cdc14 sequentially. The substrate was purified away from kinase and phosphatase prior to addition of APCCdc20. c, Quantitation of experiments conducted as in panel b using either wild-type securin, securin-2A (T27A, S71A), securin-3A (S277A, S292A, T304A), or securin-5A as substrate. Error bars indicate standard deviation from 3 experiments. Asterisks indicate a t-test P-value of <0.05 when comparing results with and without kinase.
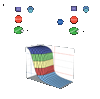
Figure 2. Modulation of securin ubiquitination by Cdk1 and Cdc14 gives rise to a potential positive feedback loop in the anaphase regulatory network
a, A simplified prior model for anaphase regulation in S. cerevisiae: the APC targets securin and some mitotic cyclins for destruction, which liberates separase to cleave cohesin and initiate anaphase. b, A modified model of anaphase control: Cdk1 phosphorylates securin, reducing the rate at which it is ubiquitinated by the APC. Upon accumulation of high levels of APC activity, some securin is destroyed, releasing a small amount of separase. Separase activates Cdc14, which dephosphorylates securin, increasing the rate at which it is ubiquitinated by the APC. Concomitantly, some mitotic cyclins are destroyed, reducing Cdk1 activity and allowing Cdc14 to more efficiently dephosphorylate securin. c, A set of numerical solutions to a simplified mathematical model of the network in panel b (but excluding cyclin degradation by the APC), showing the steady-state levels of securin remaining (y-axis) as APC activity is varied (x-axis) when securin ubiquitination is inhibited to various degrees by phosphorylation (zaxis; see Suppl. Fig. 3 for details of model).
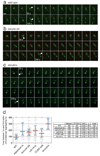
Figure 3. Modulation of securin ubiquitination by Cdk1 is required for an abrupt anaphase
a, A strain containing SPC42-dsRed:NAT; trp1∷256xLACO:TRP1; his3∷CUP1-GFP-LACI:HIS3; ura3∷112xTETO:URA3; leu2∷TETR-GFP:LEU2 has red spindle poles and GFP dots marking chromosomes IV and V. Each panel in this montage represents 10 s in a time-lapse sequence during progression through anaphase. Arrowheads indicate the 1st and 2nd chromosome separation. Each panel is a maximum intensity projection of a 7 µm stack. Note that the daughter spindle pole can be difficult to visualize due to slow maturation of the dsRed fluorophore. Scale bar is 2 µM. b, Anaphase onset in a securin-2A strain. c, Anaphase onset in a _securin_Δ strain. d, Anaphase synchrony (time between the 1st and 2nd chromosomes segregating) in approx. 40 cells of wild-type, separase mutant (esp1-1), securin-2A, cdc14-1, CLB5-Δdb, and _securin_Δ strains. The time of sister-chromatid separation was defined as the first time point when chromatids separated and did not reanneal in subsequent frames. The red line is the median and the blue bars indicate the inter-quartile range. The table at right shows the median, standard deviation and variance (standard deviation divided by the mean) for each strain. The movies corresponding to panels a–c are found in Supplementary Movies 1– 3, respectively.
Figure 4. Modulation of securin ubiquitination by Cdk1 helps coordinate anaphase onset with changes in spindle dynamics
a, Representative kymographs from wild-type and mutant cells. The x-axis is a one dimensional projection along the spindle axis (scale bar is 5 microns) and the y-axis is time (scale bar is 5 min, total time is 30 min). b, Measurements of spindle length (red diamonds) and chromosome segregation (green dots, chromosome IV; blue circles, chromosome V) in a wild-type anaphase, using a spot-tracking algorithm . Rates of the two phases of spindle elongation are highlighted by red dashed lines; initial rates of chromatid segregation are highlighted with green and blue dashed lines. When the daughter spindle pole was difficult to detect because of the slow maturation of the dsRed fluorophore, the position of the associated sister chromatid was used as a proxy for spindle pole position after anaphase A. c, Average rates of spindle elongation and sister-chromatid separation in wild-type and mutant cells. Error bars indicate standard deviation (n = 10 cells). Asterisks indicate a t-test P-value of <0.05 when comparing the wild type to the mutant. d, Rate of chromosome mis-segregation in wild-type and mutant cells. Only cells with more than the expected number of GFP dots were scored as a mis-segregation event.
Comment in
- Systems biology: On the cell cycle and its switches.
Santos SD, Ferrell JE. Santos SD, et al. Nature. 2008 Jul 17;454(7202):288-9. doi: 10.1038/454288a. Nature. 2008. PMID: 18633407 Free PMC article.
Similar articles
- Cdk1 phosphorylation of Esp1/Separase functions with PP2A and Slk19 to regulate pericentric Cohesin and anaphase onset.
Lianga N, Doré C, Kennedy EK, Yeh E, Williams EC, Fortinez CM, Wang A, Bloom KS, Rudner AD. Lianga N, et al. PLoS Genet. 2018 Mar 21;14(3):e1007029. doi: 10.1371/journal.pgen.1007029. eCollection 2018 Mar. PLoS Genet. 2018. PMID: 29561844 Free PMC article. - The yeast APC/C subunit Mnd2 prevents premature sister chromatid separation triggered by the meiosis-specific APC/C-Ama1.
Oelschlaegel T, Schwickart M, Matos J, Bogdanova A, Camasses A, Havlis J, Shevchenko A, Zachariae W. Oelschlaegel T, et al. Cell. 2005 Mar 25;120(6):773-88. doi: 10.1016/j.cell.2005.01.032. Cell. 2005. PMID: 15797379 - Mitotic exit in two dimensions.
Tóth A, Queralt E, Uhlmann F, Novák B. Tóth A, et al. J Theor Biol. 2007 Oct 7;248(3):560-73. doi: 10.1016/j.jtbi.2007.06.014. Epub 2007 Jun 17. J Theor Biol. 2007. PMID: 17659305 - Assembling the spindle midzone in the right place at the right time.
Khmelinskii A, Schiebel E. Khmelinskii A, et al. Cell Cycle. 2008 Feb 1;7(3):283-6. doi: 10.4161/cc.7.3.5349. Epub 2007 Nov 21. Cell Cycle. 2008. PMID: 18235228 Review. - How do so few control so many?
Nasmyth K. Nasmyth K. Cell. 2005 Mar 25;120(6):739-46. doi: 10.1016/j.cell.2005.03.006. Cell. 2005. PMID: 15797376 Review.
Cited by
- Nur1 dephosphorylation confers positive feedback to mitotic exit phosphatase activation in budding yeast.
Godfrey M, Kuilman T, Uhlmann F. Godfrey M, et al. PLoS Genet. 2015 Jan 8;11(1):e1004907. doi: 10.1371/journal.pgen.1004907. eCollection 2015 Jan. PLoS Genet. 2015. PMID: 25569132 Free PMC article. - APC/C-Cdh1-dependent anaphase and telophase progression during mitotic slippage.
Toda K, Naito K, Mase S, Ueno M, Uritani M, Yamamoto A, Ushimaru T. Toda K, et al. Cell Div. 2012 Feb 9;7:4. doi: 10.1186/1747-1028-7-4. Cell Div. 2012. PMID: 22321970 Free PMC article. - Bistable switches as integrators and actuators during cell cycle progression.
Stallaert W, Kedziora KM, Chao HX, Purvis JE. Stallaert W, et al. FEBS Lett. 2019 Oct;593(20):2805-2816. doi: 10.1002/1873-3468.13628. Epub 2019 Oct 16. FEBS Lett. 2019. PMID: 31566708 Free PMC article. Review. - Constraint-based analysis of gene interactions using restricted boolean networks and time-series data.
Higa CH, Louzada VH, Andrade TP, Hashimoto RF. Higa CH, et al. BMC Proc. 2011 May 28;5 Suppl 2(Suppl 2):S5. doi: 10.1186/1753-6561-5-S2-S5. BMC Proc. 2011. PMID: 21554763 Free PMC article. - Pituitary tumor transforming gene-1 haplotypes and risk of pituitary adenoma: a case-control study.
Chen S, Xiao L, Liu Z, Liu J, Liu Y. Chen S, et al. BMC Med Genet. 2011 Mar 25;12:44. doi: 10.1186/1471-2350-12-44. BMC Med Genet. 2011. PMID: 21439054 Free PMC article.
References
- Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–565. - PubMed
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. - PubMed
- Thornton BR, Toczyski DP. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20:3069–3078. - PubMed
- Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8:894–903. - PubMed
- Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous