Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade - PubMed (original) (raw)
. 2008 Jun 24;105(25):8754-9.
doi: 10.1073/pnas.0710823105. Epub 2008 Jun 16.
Thomas A Comery, Robert L Martone, Hassan Elokdah, David L Crandall, Aram Oganesian, Suzan Aschmies, Yolanda Kirksey, Cathleen Gonzales, Jane Xu, Hua Zhou, Kevin Atchison, Erik Wagner, Margaret M Zaleska, Indranil Das, Robert L Arias, Jonathan Bard, David Riddell, Stephen J Gardell, Magid Abou-Gharbia, Albert Robichaud, Ronald Magolda, George P Vlasuk, Thorir Bjornsson, Peter H Reinhart, Menelas N Pangalos
Affiliations
- PMID: 18559859
- PMCID: PMC2438386
- DOI: 10.1073/pnas.0710823105
Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade
J Steven Jacobsen et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The amyloid hypothesis states that a variety of neurotoxic beta-amyloid (Abeta) species contribute to the pathogenesis of Alzheimer's disease. Accordingly, a key determinant of disease onset and progression is the appropriate balance between Abeta production and clearance. Enzymes responsible for the degradation of Abeta are not well understood, and, thus far, it has not been possible to enhance Abeta catabolism by pharmacological manipulation. We provide evidence that Abeta catabolism is increased after inhibition of plasminogen activator inhibitor-1 (PAI-1) and may constitute a viable therapeutic approach for lowering brain Abeta levels. PAI-1 inhibits the activity of tissue plasminogen activator (tPA), an enzyme that cleaves plasminogen to generate plasmin, a protease that degrades Abeta oligomers and monomers. Because tPA, plasminogen and PAI-1 are expressed in the brain, we tested the hypothesis that inhibitors of PAI-1 will enhance the proteolytic clearance of brain Abeta. Our data demonstrate that PAI-1 inhibitors augment the activity of tPA and plasmin in hippocampus, significantly lower plasma and brain Abeta levels, restore long-term potentiation deficits in hippocampal slices from transgenic Abeta-producing mice, and reverse cognitive deficits in these mice.
Conflict of interest statement
Conflict of interest statement: All coauthors are current or former employees of Wyeth Research.
Figures
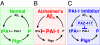
Fig. 1.
Aβ activates and is cleaved by the tPA/plasmin cascade. Schematic representation to demonstrate the proteolytic clearance of β-amyloid (Aβ) in a cascade including tissue plasminogen activator (tPA), plasmin (P), and the tPA inhibitor plasminogen activator inhibitor-1 (PAI-1). (A) Normal. Aβ monomers aggregate into oligomers and/or fibrils; Aβ aggregates (Aβn) induce the expression of tPA and enhance the activation of tPA; and tPA cleaves plasminogen (Plgn) to liberate active plasmin, which cleaves Aβ. (B) Alzheimer's disease. PAI-1 binds to tPA inhibiting its activity and preventing the activation of plasmin and the proteolytic clearance of Aβ. (C) Treatment with PAI-1 inhibitor. The small-molecule inhibitor of PAI-1, PAZ-417, prevents formation of the PAI-1/tPA complex, resulting in sustained proteolytic tPA activity, activation of plasmin, and the proteolytic clearance of Aβ.
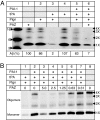
Fig. 2.
A small-molecule inhibitor of PAI-1 results in cleavage of monomeric and oligomeric Aβ42 in an in vitro assay. Aβ42 peptide cleavage was assessed by Western blot after in vitro incubation with recombinant human PAI-1 and purified tPA and plasminogen proteins as described (see
SI Text
). (A) Cleavage of monomeric and oligomeric Aβ42 in response to PAZ-417. Control Aβ was incubated in assay buffers (lane 1), with plasminogen alone (lane 2), with tPA alone (lane 4), or with tPA and plasminogen (lane 3). Only the combination of both tPA and plasminogen resulted in cleavage (98%) of monomeric and oligomeric Aβ. Cleavage of Aβ was inhibited with the preincubation of PAI-1 and subsequent addition of plasminogen (lane 5) but is restored by 93% when the mixture is preincubated with 5 μM PAZ-417 (lane 6). Indicated are the molecular weight markers (on the left); positions of Aβ monomer, dimer, trimer, and tetramer (on the right); and the percentage of remaining monomeric Aβ (Lower). (B) Dose-dependent cleavage of monomeric and oligomeric Aβ42 in response to PAZ-417. Control for maximal cleavage Aβ (tPA and plasminogen, no PAI-1, lane 1); control for minimal cleavage of Aβ (PAI-1, tPA, plasminogen and vehicle, lane 2); and dose-response with decreasing treatment with PAZ-417 at 5, 2.5, 1.25, 0.63, and 0.31 μM (lanes 3–7); untreated Aβ control displaying starting levels of monomer and oligomers (lane 8).
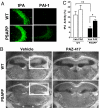
Fig. 3.
A small-molecule inhibitor of PAI-1 restores tPA activity in the hippocampus of PSAPP mice. (A) Immunohistochemical analysis demonstrating protein levels of tPA (Left) and PAI-1 (Right) in the hippocampal CA2/CA3 brain region of WT and PSAPP mice. (B) Representative zymograms highlighting regions of the brain containing tPA protease activity visualized by dark-field micrographs shown for each genotype and treatment. Areas of dark exposure (example indicated by white boxes) reflect zones of substrate hydrolysis localizing tPA protease activity. (C) Quantitative analysis of tPA activity measured from zymograms of hippocampal brain regions (n = 5). The areas of tPA-associated lysis visualized by dark-field illumination are expressed as percentages of the area of hippocampus in the same plane (*, P < 0.004; **, P < 0.04).
Fig. 4.
A small-molecule inhibitor of PAI-1 reduces plasma and brain Aβ levels in transgenic APP mice. (A) Time-course of plasma Aβ40 lowering measured in response to a single administration of PAZ-417 (100 mg/kg, po) or vehicle at the indicated posttreatment times (*, P < 0.005; **, P < 0.001) to Tg2576 mice. (B) Dose-response of PAZ-417 on Aβ40 lowering at 6 h after treatment (*, P < 0.02). (C and D) Plasma Aβ40 or brain Aβ40 and Aβ42 levels after a single administration of PAZ-417 (20 mg/kg, po) 6 h after treatment to (C) Tg2576 or (D) PSAPP mice (*, P < 0.01; **, P < 0.001). Aβ levels are presented as percentages (%) of vehicle treatment.
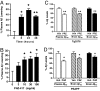
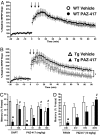
Fig. 5.
PAZ-417 reduces both hippocampal LTP and contextual memory deficits in Tg2576 mice. (A) Administration of PAZ-417 (100 mg/kg, po) to WT mice (21–25 weeks of age) 24 h before hippocampal slice preparation has no effect on LTP induced in the dentate gyrus (DG) by high-frequency stimulation of the perforant path. (B) In contrast, when similarly administered to age-matched Tg2576 (Tg) mice, PAZ-417 restored DG LTP to WT levels (P < 0.05). (C) Dose-dependent effects of drug on reversing contextual memory deficits in Tg2576 and WT mice after administration of a single dose of PAZ-417 (10, 30 or 100 mg/kg, po) 4 h before training or of DAPT (100 mg/kg, po) 3 h before training. Transgenic animals exhibited significantly reduced contextual memory compared with WT mice (*, P < 0.002). Drug-treated transgenic animals exhibited significantly improved contextual memory compared with vehicle-treated transgenic animals (#, P < 0.02). (D) Time-dependent effects of PAZ-417 on reversing contextual memory deficits in Tg2576 and WT mice over a 24 h period after administration of a single dose (10 mg/kg, po). Transgenic animals exhibited significantly reduced contextual memory compared with WT mice (*, P < 0.002). Drug-treated transgenic animals exhibited significantly improved contextual memory compared with vehicle-treated transgenic animals (#, P < 0.0001). Twenty week-old Tg2576 (filled bars) and WT (open bars) mice.
Similar articles
- Impacts of aging and amyloid-β deposition on plasminogen activators and plasminogen activator inhibitor-1 in the Tg2576 mouse model of Alzheimer's disease.
Bi Oh S, Suh N, Kim I, Lee JY. Bi Oh S, et al. Brain Res. 2015 Feb 9;1597:159-67. doi: 10.1016/j.brainres.2014.11.042. Epub 2014 Nov 29. Brain Res. 2015. PMID: 25454795 - The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration.
Melchor JP, Pawlak R, Strickland S. Melchor JP, et al. J Neurosci. 2003 Oct 1;23(26):8867-71. doi: 10.1523/JNEUROSCI.23-26-08867.2003. J Neurosci. 2003. PMID: 14523088 Free PMC article. - The probable role of tissue plasminogen activator/neuroserpin axis in Alzheimer's disease: a new perspective.
Ali NH, Al-Kuraishy HM, Al-Gareeb AI, Alnaaim SA, Alexiou A, Papadakis M, Saad HM, Batiha GE. Ali NH, et al. Acta Neurol Belg. 2024 Apr;124(2):377-388. doi: 10.1007/s13760-023-02403-x. Epub 2023 Nov 2. Acta Neurol Belg. 2024. PMID: 37917293 Free PMC article. Review. - The plasmin system is induced by and degrades amyloid-beta aggregates.
Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, Walker D, Scheff S, McGillis JP, Rydel RE, Estus S. Tucker HM, et al. J Neurosci. 2000 Jun 1;20(11):3937-46. doi: 10.1523/JNEUROSCI.20-11-03937.2000. J Neurosci. 2000. PMID: 10818128 Free PMC article. - Fibrinolysis: the key to new pathogenetic mechanisms.
Zorio E, Gilabert-Estellés J, España F, Ramón LA, Cosín R, Estellés A. Zorio E, et al. Curr Med Chem. 2008;15(9):923-9. doi: 10.2174/092986708783955455. Curr Med Chem. 2008. PMID: 18473800 Review.
Cited by
- Genetic association of urokinase-type plasminogen activator gene rs2227564 site polymorphism with sporadic Alzheimer's disease in the Han Chinese population.
Ji X, Jia L, Jia J, Qi L. Ji X, et al. Neural Regen Res. 2012 Oct 25;7(30):2377-83. doi: 10.3969/j.issn.1673-5374.2012.30.008. Neural Regen Res. 2012. PMID: 25538763 Free PMC article. - The islet tissue plasminogen activator/plasmin system is upregulated with human islet amyloid polypeptide aggregation and protects beta cells from aggregation-induced toxicity.
Esser N, Hogan MF, Templin AT, Akter R, Fountaine BS, Castillo JJ, El-Osta A, Manathunga L, Zhyvoloup A, Raleigh DP, Zraika S, Hull RL, Kahn SE. Esser N, et al. Diabetologia. 2024 Sep;67(9):1897-1911. doi: 10.1007/s00125-024-06161-0. Epub 2024 Sep 9. Diabetologia. 2024. PMID: 39245780 Free PMC article. - Targeting Abeta and tau in Alzheimer's disease, an early interim report.
Golde TE, Petrucelli L, Lewis J. Golde TE, et al. Exp Neurol. 2010 Jun;223(2):252-66. doi: 10.1016/j.expneurol.2009.07.035. Epub 2009 Aug 27. Exp Neurol. 2010. PMID: 19716367 Free PMC article. Review. - Powering Amyloid Beta Degrading Enzymes: A Possible Therapy for Alzheimer's Disease.
Sikanyika NL, Parkington HC, Smith AI, Kuruppu S. Sikanyika NL, et al. Neurochem Res. 2019 Jun;44(6):1289-1296. doi: 10.1007/s11064-019-02756-x. Epub 2019 Feb 26. Neurochem Res. 2019. PMID: 30806879 Review. - Small-molecule activators of insulin-degrading enzyme discovered through high-throughput compound screening.
Cabrol C, Huzarska MA, Dinolfo C, Rodriguez MC, Reinstatler L, Ni J, Yeh LA, Cuny GD, Stein RL, Selkoe DJ, Leissring MA. Cabrol C, et al. PLoS One. 2009;4(4):e5274. doi: 10.1371/journal.pone.0005274. Epub 2009 Apr 22. PLoS One. 2009. PMID: 19384407 Free PMC article.
References
- Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
- Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. - PubMed
- Selkoe DJ. Clearing the brain's amyloid cobwebs. Neuron. 2001;32:177–180. - PubMed
- Selkoe DJ. Alzheimer disease: Mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. - PubMed
- Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann NY Acad Sci. 2000;924:17–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous