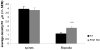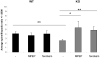Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice - PubMed (original) (raw)
Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice
Femke M S de Vrij et al. Neurobiol Dis. 2008 Jul.
Abstract
Lack of fragile X mental retardation protein (FMRP) causes Fragile X Syndrome, the most common form of inherited mental retardation. FMRP is an RNA-binding protein and is a component of messenger ribonucleoprotein complexes, associated with brain polyribosomes, including dendritic polysomes. FMRP is therefore thought to be involved in translational control of specific mRNAs at synaptic sites. In mice lacking FMRP, protein synthesis-dependent synaptic plasticity is altered and structural malformations of dendritic protrusions occur. One hypothesized cause of the disease mechanism is based on exaggerated group I mGluR receptor activation. In this study, we examined the effect of the mGluR5 antagonist MPEP on Fragile X related behavior in Fmr1 KO mice. Our results demonstrate a clear defect in prepulse inhibition of startle in Fmr1 KO mice, that could be rescued by MPEP. Moreover, we show for the first time a structural rescue of Fragile X related protrusion morphology with two independent mGluR5 antagonists.
Figures
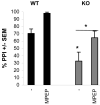
Fig.1. Rescue of prepulse inhibition of startle in Fmr1 KO mice
Both wild type and Fmr1 KO mice were subjected to prepulse inhibition of startle procedures. Fmr1 KO mice displayed a dramatic impairment of PPI on day 1 (baseline levels). This reduction was rescued to wild type levels on day 2 by injection of 20 mg/kg MPEP 30 minutes prior to training. Interestingly, the wild types showed an equal improvement of PPI performance after injection of MPEP.
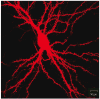
Fig.2
Representative image of a wild type E18 hippocampal mouse neuron (DIV21), transfected with a β-actin-mCherry construct.
Fig.3. Dendrite branching is normal in Fmr1 KO primary hippocampal neurons
Sholl analysis of wild type and Fmr1 KO primary hippocampal neurons cultured in parallel was performed with Metamorph software. Average of three independent experiments.
Fig.4. Fmr1 KO primary hippocampal neurons have an immature protrusion phenotype
Protrusion densities of wild type and Fmr1 KO primary hippocampal neurons cultured in parallel were counted with Metamorph software. Fmr1 KO neurons had significantly more filopodia than wild type neurons (p<0,001), corresponding to an immature phenotype. Averages of 3 independent experiments, compared with Student's T tests. The distinction between spines and filopodia was made objectively by using a threshold ratio of 0,5 for the width/length ratio of protrusions.
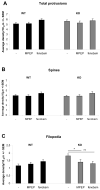
Fig.5. Rescue of protrusion morphology in Fmr1 KO primary hippocampal neurons
Fmr1 KO and wild type neurons were treated for four hours with 200 μm MPEP or 300 μm fenobam. The total amount of protrusions (A) and the amount of mature spines (B) were unaffected by mGluR5 antagonist treatment. The Fmr1 KO phenotype showing an increased number of filopodia was completely rescued by both mGluR5 antagonists (C). Averages of 3 independent experiments, compared with Student's T tests (*=p<0,05, **=p<0,01).
Fig.6. mGluR5 antagonist treatment changes the distribution of spines and filopodia in Fmr1 KO neurons
The average spine/filopodia ratio changes significantly in Fmr1 KO primary hippocampal neurons after treatment with two independent mGluR5 antagonists. As total protrusion density is not different between wild type and Fmr1 KO neurons, we can conclude that the excess of filopodia in Fmr1 KO neurons can successfully be changed into or replaced by spines.
Similar articles
- Involvement of Phosphodiesterase 2A Activity in the Pathophysiology of Fragile X Syndrome.
Maurin T, Melancia F, Jarjat M, Castro L, Costa L, Delhaye S, Khayachi A, Castagnola S, Mota E, Di Giorgio A, Servadio M, Drozd M, Poupon G, Schiavi S, Sardone L, Azoulay S, Ciranna L, Martin S, Vincent P, Trezza V, Bardoni B. Maurin T, et al. Cereb Cortex. 2019 Jul 22;29(8):3241-3252. doi: 10.1093/cercor/bhy192. Cereb Cortex. 2019. PMID: 30137253 - Functional rescue of excitatory synaptic transmission in the developing hippocampus in Fmr1-KO mouse.
Meredith RM, de Jong R, Mansvelder HD. Meredith RM, et al. Neurobiol Dis. 2011 Jan;41(1):104-10. doi: 10.1016/j.nbd.2010.08.026. Epub 2010 Sep 15. Neurobiol Dis. 2011. PMID: 20817093 - Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome.
Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. Thomas AM, et al. Psychopharmacology (Berl). 2012 Jan;219(1):47-58. doi: 10.1007/s00213-011-2375-4. Epub 2011 Jun 10. Psychopharmacology (Berl). 2012. PMID: 21656124 - Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development.
Pop AS, Gomez-Mancilla B, Neri G, Willemsen R, Gasparini F. Pop AS, et al. Psychopharmacology (Berl). 2014 Mar;231(6):1217-26. doi: 10.1007/s00213-013-3330-3. Psychopharmacology (Berl). 2014. PMID: 24232444 Review. - BDNF in fragile X syndrome.
Castrén ML, Castrén E. Castrén ML, et al. Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
Cited by
- Differential effects by sex with Kmt5b loss.
Wickramasekara RN, Robertson B, Hulen J, Hallgren J, Stessman HAF. Wickramasekara RN, et al. Autism Res. 2021 Aug;14(8):1554-1571. doi: 10.1002/aur.2516. Epub 2021 Apr 19. Autism Res. 2021. PMID: 33871180 Free PMC article. - Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model.
Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Goebel-Goody SM, et al. Genes Brain Behav. 2012 Jul;11(5):586-600. doi: 10.1111/j.1601-183X.2012.00781.x. Epub 2012 Apr 6. Genes Brain Behav. 2012. PMID: 22405502 Free PMC article. - A Two-Hit Approach Inducing Flurothyl Seizures in Fmr1 Knockout Mice Impacts Anxiety and Repetitive Behaviors.
Blandin KJ, Narvaiz DA, Sullens DG, Womble PD, Hodges SL, Binder MS, Faust A, Nguyen PH, Pranske ZJ, Lugo JN. Blandin KJ, et al. Brain Sci. 2024 Aug 31;14(9):892. doi: 10.3390/brainsci14090892. Brain Sci. 2024. PMID: 39335388 Free PMC article. - Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex.
Fatemi SH, Folsom TD, Kneeland RE, Yousefi MK, Liesch SB, Thuras PD. Fatemi SH, et al. Mol Autism. 2013 Jun 26;4(1):21. doi: 10.1186/2040-2392-4-21. Mol Autism. 2013. PMID: 23803181 Free PMC article. - Therapeutic potential of metabotropic glutamate receptor modulators.
Hovelsø N, Sotty F, Montezinho LP, Pinheiro PS, Herrik KF, Mørk A. Hovelsø N, et al. Curr Neuropharmacol. 2012 Mar;10(1):12-48. doi: 10.2174/157015912799362805. Curr Neuropharmacol. 2012. PMID: 22942876 Free PMC article.
References
- Antar LN, et al. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–9. - PubMed
- Antar LN, et al. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. - PubMed
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–87. - PubMed
- Bakker CE, et al. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. - PubMed
- Bardoni B, et al. The fragile X syndrome: exploring its molecular basis and seeking a treatment. Expert Rev Mol Med. 2006;8:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD38038/HD/NICHD NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- R01 HD038038/HD/NICHD NIH HHS/United States
- R01 HD038038-05/HD/NICHD NIH HHS/United States
- P30 HD024064-19/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials