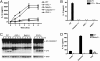The Nalp3 inflammasome is essential for the development of silicosis - PubMed (original) (raw)
The Nalp3 inflammasome is essential for the development of silicosis
Suzanne L Cassel et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Inhalation of crystalline silica and asbestos is known to cause the progressive pulmonary fibrotic disorders silicosis and asbestosis, respectively. Although alveolar macrophages are believed to initiate these inflammatory responses, the mechanism by which this occurs has been unclear. Here we show that the inflammatory response and subsequent development of pulmonary fibrosis after inhalation of silica is dependent on the Nalp3 inflammasome. Stimulation of macrophages with silica results in the activation of caspase-1 in a Nalp3-dependent manner. Macrophages deficient in components of the Nalp3 inflammasome were incapable of secreting the proinflammatory cytokines interleukin (IL)-1beta and IL-18 in response to silica. Similarly, asbestos was capable of activating caspase-1 in a Nalp3-dependent manner. Activation of the Nalp3 inflammasome by silica required both an efflux of intracellular potassium and the generation of reactive oxygen species. This study demonstrates a key role for the Nalp3 inflammasome in the pathogenesis of pneumoconiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
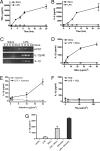
Fig. 1.
Silica induces IL-1β secretion from LPS-primed macrophages. (_A_–C) Macrophages from WT mice were stimulated with either 50 μg/cm2 silica or 50 ng/ml LPS. Culture supernatants were collected at the indicated time after stimulation; TNFα (A) and IL-6 (B) release into culture supernatants was measured by ELISA. (C) IL-1β and IL-12p40 expression in silica- or LPS-stimulated macrophages was determined by RT-PCR. (D–F) Macrophages from WT mice were either primed with 50 ng/ml LPS or left untreated. Macrophages were stimulated with the indicated dose of silica (D), asbestos (E), or titanium dioxide (F), and culture supernatants were collected 4 h later. IL-1β release into culture supernatants was measured by ELISA. (G) Human alveolar macrophages were either primed with 50 ng/ml LPS or left untreated. Macrophages were stimulated with 50 μg/cm2 silica or 40 μg/cm2 asbestos for 4 h, and IL-1β release into culture supernatants was measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of two separate experiments.
Fig. 2.
Silica- and asbestos-induced IL-1β secretion is dependent on Nalp3. LPS-primed macrophages from WT, caspase-1-, ASC-, Nalp3-, or IPAF-deficient mice were stimulated with 50 μg/cm2 silica. Culture supernatants were collected at 4 h or at the indicated time after stimulation (A–C) and IL-1β and IL-18 release into culture supernatants was measured by ELISA (A and B). Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of three (A) and two (B) separate experiments. (C) Lysates from LPS-primed WT, ASC-, NALP3-, or IPAF-deficient macrophages stimulated with 50 μg/cm2 silica for the indicated times were immunoblotted with antibodies against the p10 subunit of caspase-1. (D) LPS-primed macrophages from WT and NALP3-deficient mice were stimulated with either 40 μg/cm2 asbestos or 50 μg/cm2 titanium dioxide. Culture supernatants were collected 6 h later and IL-1β release into culture supernatants was measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of three separate experiments. *, Nondetectable.
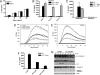
Fig. 3.
Silica-induced IL-1β secretion requires internalization of silica, a potassium efflux, and generation of reactive oxygen species. (A) LPS-primed WT macrophages were stimulated with the indicated amount of silica. Culture supernatants were collected 4 h later and cytotoxicity was measured by lactate dehydrogenase (LDH) release and expressed as a percentage of LDH release by Triton X-100 detergent. (B) LPS-primed WT macrophages were incubated with 10 μM cytochalasin B or 20 μM cytochalasin D for 10 min before the addition of 50 μg/cm2 silica or 5 mM ATP. (C) LPS-primed macrophages were incubated in either high Na+- or high K+-containing medium and then stimulated with either 50 μg/cm2 silica or 5 mM ATP. ATP-treated cells had their medium replaced with fresh medium after 20 min. Four hours after initial stimulation, culture supernatants were collected and IL-1β release was measured by ELISA. (A–C) Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of two separate experiments. (D and E) WT and Nalp3−/− macrophages were stimulated with the indicated amount of silica, and ROS production was measured by luminol-amplified chemiluminescence. RLU, relative light units. (F and G) LPS-primed WT macrophages were incubated with 10 μM or the indicated amount of diphenyleneiodonium chloride (DPI) for 10 min before the addition of 50 μg/cm2 silica. Four hours after initial stimulation, culture supernatants were collected and IL-1β release was measured by ELISA (F). Determinations were performed in triplicate and expressed as the mean ± SEM and are representative of three separate experiments. At the indicated time after stimulation lysates were collected and immunoblotted with antibodies against the p10 subunit of caspase-1, caspase-3, and actin (G). *, Nondetectable.
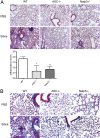
Fig. 4.
Decreased inflammation and collagen deposition in ASC- and Nalp3-deficient mice after intranasal instillation of silica. WT, ASC−/−, and Nalp3−/− mice received either intranasal PBS or silica. Three months after the initial challenge, lungs were stained with H&E to examine inflammation (A) or trichrome stain to examine collagen deposition (B). H&E-stained sections were scored in a blinded fashion for cellular tissue infiltration (*, P = 0.039; **, P = 0.0062 compared with WT by one-tailed unpaired Student's t test). Images were obtained at ×10 (A) and ×20 (B) magnification and are representative of two mice per group for PBS and five mice per group for silica.
Similar articles
- Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization.
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Hornung V, et al. Nat Immunol. 2008 Aug;9(8):847-56. doi: 10.1038/ni.1631. Epub 2008 Jul 11. Nat Immunol. 2008. PMID: 18604214 Free PMC article. - Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration.
Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Pétrilli V, et al. Cell Death Differ. 2007 Sep;14(9):1583-9. doi: 10.1038/sj.cdd.4402195. Epub 2007 Jun 29. Cell Death Differ. 2007. PMID: 17599094 - Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid.
Griffith JW, Sun T, McIntosh MT, Bucala R. Griffith JW, et al. J Immunol. 2009 Oct 15;183(8):5208-20. doi: 10.4049/jimmunol.0713552. Epub 2009 Sep 25. J Immunol. 2009. PMID: 19783673 Free PMC article. - Silica-induced inflammasome activation in macrophages: role of ATP and P2X7 receptor.
Luna-Gomes T, Santana PT, Coutinho-Silva R. Luna-Gomes T, et al. Immunobiology. 2015 Sep;220(9):1101-6. doi: 10.1016/j.imbio.2015.05.004. Epub 2015 May 18. Immunobiology. 2015. PMID: 26024943 Review. - ASC, Ipaf and Cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome.
Mariathasan S. Mariathasan S. Microbes Infect. 2007 Apr;9(5):664-71. doi: 10.1016/j.micinf.2007.01.017. Epub 2007 Jan 27. Microbes Infect. 2007. PMID: 17382568 Review.
Cited by
- GLP-1R activation attenuates the progression of pulmonary fibrosis via disrupting NLRP3 inflammasome/PFKFB3-driven glycolysis interaction and histone lactylation.
Liu C, Zhang Q, Zhou H, Jin L, Liu C, Yang M, Zhao X, Ding W, Xie W, Kong H. Liu C, et al. J Transl Med. 2024 Oct 21;22(1):954. doi: 10.1186/s12967-024-05753-z. J Transl Med. 2024. PMID: 39434134 Free PMC article. - Inflammasome Activity in Non-Microbial Lung Inflammation.
Ather JL, Martin RA, Ckless K, Poynter ME. Ather JL, et al. J Environ Immunol Toxicol. 2014 Sep 20;1(3):108-117. J Environ Immunol Toxicol. 2014. PMID: 25642415 Free PMC article. - Materials design at the interface of nanoparticles and innate immunity.
Szeto GL, Lavik EB. Szeto GL, et al. J Mater Chem B. 2016 Mar 7;4(9):1610-1618. doi: 10.1039/C5TB01825K. Epub 2016 Jan 29. J Mater Chem B. 2016. PMID: 27453783 Free PMC article. - Lysosomal BK channels facilitate silica-induced inflammation in macrophages.
Kendall RL, Holian A. Kendall RL, et al. Inhal Toxicol. 2024 Jan;36(1):31-43. doi: 10.1080/08958378.2024.2305112. Epub 2024 Jan 23. Inhal Toxicol. 2024. PMID: 38261520 - Molecular Evaluation of the IFN γ +874, TNF α -308, and IL-1Ra VNTR Sequences in Silicosis.
Rad IA, Mohebbi I, Bagheri M. Rad IA, et al. Maedica (Bucur). 2012 Jan;7(1):20-4. Maedica (Bucur). 2012. PMID: 23118815 Free PMC article.
References
- Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med. 1998;157:1666–1680. - PubMed
- Otsuki T, et al. Immunological effects of silica and asbestos. Cell Mol Immunol. 2007;4:261–268. - PubMed
- Wagner GR. Asbestosis and silicosis. Lancet. 1997;349:1311–1315. - PubMed
- Kline JN, Schwartz DA, Monick MM, Floerchinger CS, Hunninghake GH. Relative release of interleukin-1β and interleukin-1 receptor antagonist by alveolar macrophages. A study in asbestos-induced lung disease, sarcoidosis, and idiopathic pulmonary fibrosis. Chest. 1993;104:47–53. - PubMed
- Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced interleukin-1β and tumor necrosis factor-α release and mRNA expression in macrophages from idiopathic pulmonary fibrosis or following asbestos exposure. J Immunol. 1993;150:4188–4196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES015981/ES/NIEHS NIH HHS/United States
- ES014871/ES/NIEHS NIH HHS/United States
- T32 AR007016/AR/NIAMS NIH HHS/United States
- R01 ES015981/ES/NIEHS NIH HHS/United States
- T32 HL007974/HL/NHLBI NIH HHS/United States
- K08 AI067736/AI/NIAID NIH HHS/United States
- K08 AI065517/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 ES014871/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous