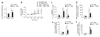Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins - PubMed (original) (raw)
Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins
Jingsong Xu et al. Nat Immunol. 2008 Aug.
Abstract
Nonmuscle myosin light-chain kinase (MYLK) mediates increased lung vascular endothelial permeability in lipopolysaccharide-induced lung inflammatory injury, the chief cause of the acute respiratory distress syndrome. In a lung injury model, we demonstrate here that MYLK was also essential for neutrophil transmigration, but that this function was mostly independent of myosin II regulatory light chain, the only known substrate of MYLK. Instead, MYLK in neutrophils was required for the recruitment and activation of the tyrosine kinase Pyk2, which mediated full activation of beta(2) integrins. Our results demonstrate that MYLK-mediated activation of beta(2) integrins through Pyk2 links beta(2) integrin signaling to the actin motile machinery of neutrophils.
Figures
Figure 1. Ex vivo LPS-induced lung injury and edema formation
(a) Change in microvessel permeability in wild-type lungs after perfusion of wild-type (WT) or _Mylk_−/− neutrophils (PMN), followed by challenge for 16 h with LPS (10 mg/kg given intraperitoneally), assessed as the pulmonary microvessel filtration coefficient (_K_fc) and presented as per gram of dry lung weight (dry g). (b) Time-dependent change in pulmonary edema formation, as assessed by the increase in wet weight of lungs treated as described in a. (c) Sequestration of 111In-oxine–labeled PMNs in lungs after 111In-labeled PMNs were stimulated with LPS or fMLP and then added to perfusates of lung preparations. (d) Adhesion of wild-type and _Mylk_−/− PMNs to cultured mouse lung vascular endothelial cells. (e) Transmigration of LPS-primed wild-type and _Mylk_−/− PMNs across endothelial cells toward fMLP in the lower chamber of a Transwell. (f) Migration of PMNs into the airspace of wild-type and _Mylk_−/− mice challenged with LPS. *, P < 0.05, compared with basal; **, P < 0.05, compared with wild type after LPS stimulation. Data are the mean of five (a,b), four (c–e) or six (f) independent experiments (error bars, s.e.m.).
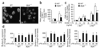
Figure 2. Loss of MYLK function fails to prevent myosin II activation
(a,b) MLC phosphorylation in wild-type and _Mylk_−/− lungs (a) or PMNs (b). Bottom blots, confirmation of equal protein loading by analysis with anti-MLC. Above, densitometry analysis. *, P < 0.05, compared with basal (no LPS stimulation). Data are from one experiment representative of three (error bars, s.e.m.). (c) Subcellular distribution of activated myosin II (top; anti-p19-MLC) and MYLK (bottom; anti-MYLK) in polarized PMNs. Arrows indicate leading edges. Scale bars, 10 µm. Results are representative of three experiments. (d) Adhesion of wild-type PMNs preincubated with LPS (1 µg/ml), then cultured for 30 min at 37 °C together with mouse lung vascular endothelial cells with or without inhibitors (horizontal axis). Blebbi, blebbistatin. *, P < 0.05, compared with basal; **, P < 0.05, treatment with inhibitor plus LPS compared with LPS alone. Data are the mean of four independent experiments (error bars, s.e.m.).
Figure 3. Activation of β2 integrin in neutrophils
(a) Microscopy of wild-type and _Mylk_−/− PMNs spread on ICAM-1–coated surfaces, then fixed with methanol and stained with anti-aM. Original magnification, ×60. (b) Adhesion to ICAM-1–coated surfaces of wild-type and _Mylk_−/− PMNs challenged with LPS or not. *, P < 0.05, compared with no LPS; **, P < 0.05, compared with wild type after LPS stimulation. (c) Analysis of β2 integrin activation by soluble ICAM-1 binding assay with (+) or without (−) 10 mM Mn2+. ICAM-1–Fc, Fc-tagged ICAM-1. *, P < 0.05, compared with no stimulation; **, P < 0.05, compared with wild type after Mn2+ stimulation. (d) Flow cytometry of the surface expression of β2 integrins on circulating wild-type and _Mylk_−/− PMNs. *, P < 0.05, compared with no LPS. Data are from one experiment representative of three (a) or are the mean of four independent experiments (b–d; error bars, s.e.m.).
Figure 4. Tyrosine kinase activation and interaction with β2 integrin
(a) Phosphorylation (p-) of c-Src, Syk and Pyk2 in wild-type and _Mylk_−/− PMNs. Bottom blots, confirmation of equal protein loading by analysis with anti-c-Src, anti-Pyk2 and anti-Syk. Above, densitometry analysis. *, P < 0.05, compared with no LPS; **, P < 0.05, compared with other groups after LPS stimulation. Data are the mean of four independent experiments (error bars, s.e.m.). (b) Colocalization of MYLK and Pyk2 in wild-type PMNs stimulated with fMLP and stained with anti-MYLK and anti-Pyk2 (above). Arrows indicate leading edges. Scale bar, 10 µm. Below, coimmunoprecipitation of MYLK and Pyk2 in wild-type PMNs. IP, immunoprecipitation; IB, immunoblot. Data are from one experiment representative of three. (c) Association of β2 integrin with c-Src and Pyk2 in wild-type and _Mylk_−/− PMNs stimulated for 0, 30 or 60 min with LPS (1 µg/ml); equal amounts of protein immunoprecipitated from lysates with anti–β2 integrin are analyzed by immunoblot with anti-Pyk2, anti-c-Src or anti–β2 integrin. IgG, immunoglobulin G (control antibody). Data are representative of three independent experiments.
Figure 5. The interaction of β2 integrin and the cytoskeleton in neutrophils
Association of β2 integrin with the actin-associated proteins talin (a) and α-actinin (b) in wild-type and _Mylk_−/− PMNs stimulated for 0, 30 or 60 min with LPS (1 µg/ml); equal amounts of protein immunoprecipitated from lysates with anti–β2 integrin are analyzed by immunoblot with anti-talin or anti-α-actinin. IgG, control antibody. Data are representative of three independent experiments.
Similar articles
- Myeloid Poldip2 Contributes to the Development of Pulmonary Inflammation by Regulating Neutrophil Adhesion in a Murine Model of Acute Respiratory Distress Syndrome.
Ou Z, Dolmatova E, Mandavilli R, Qu H, Gafford G, White T, Valdivia A, Lassègue B, Hernandes MS, Griendling KK. Ou Z, et al. J Am Heart Assoc. 2022 May 17;11(10):e025181. doi: 10.1161/JAHA.121.025181. Epub 2022 May 10. J Am Heart Assoc. 2022. PMID: 35535614 Free PMC article. - Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury.
Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, Shriver MD, Ingersoll R, Scott AF, Beaty TH, Moitra J, Ma SF, Ye SQ, Barnes KC, Garcia JG. Gao L, et al. Am J Respir Cell Mol Biol. 2006 Apr;34(4):487-95. doi: 10.1165/rcmb.2005-0404OC. Epub 2006 Jan 6. Am J Respir Cell Mol Biol. 2006. PMID: 16399953 Free PMC article. - MicroRNA regulation of nonmuscle myosin light chain kinase expression in human lung endothelium.
Adyshev DM, Moldobaeva N, Mapes B, Elangovan V, Garcia JG. Adyshev DM, et al. Am J Respir Cell Mol Biol. 2013 Jul;49(1):58-66. doi: 10.1165/rcmb.2012-0397OC. Am J Respir Cell Mol Biol. 2013. PMID: 23492194 Free PMC article. - Role of β2 Integrins in Neutrophils and Sepsis.
Yuki K, Hou L. Yuki K, et al. Infect Immun. 2020 May 20;88(6):e00031-20. doi: 10.1128/IAI.00031-20. Print 2020 May 20. Infect Immun. 2020. PMID: 32205406 Free PMC article. Review. - β2 Integrin Regulation of Neutrophil Functional Plasticity and Fate in the Resolution of Inflammation.
Sekheri M, Othman A, Filep JG. Sekheri M, et al. Front Immunol. 2021 Mar 30;12:660760. doi: 10.3389/fimmu.2021.660760. eCollection 2021. Front Immunol. 2021. PMID: 33859651 Free PMC article. Review.
Cited by
- Critical role of non-muscle myosin light chain kinase in thrombin-induced endothelial cell inflammation and lung PMN infiltration.
Fazal F, Bijli KM, Murrill M, Leonard A, Minhajuddin M, Anwar KN, Finkelstein JN, Watterson DM, Rahman A. Fazal F, et al. PLoS One. 2013;8(3):e59965. doi: 10.1371/journal.pone.0059965. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555849 Free PMC article. - Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin.
Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, Chong HC, Tan SM, Loo SC, Ng KW, Xie JP, Ong CN, Tan NS, Leong DT. Setyawati MI, et al. Nat Commun. 2013;4:1673. doi: 10.1038/ncomms2655. Nat Commun. 2013. PMID: 23575677 - Moesin and myosin phosphatase confine neutrophil orientation in a chemotactic gradient.
Liu X, Yang T, Suzuki K, Tsukita S, Ishii M, Zhou S, Wang G, Cao L, Qian F, Taylor S, Oh MJ, Levitan I, Ye RD, Carnegie GK, Zhao Y, Malik AB, Xu J. Liu X, et al. J Exp Med. 2015 Feb 9;212(2):267-80. doi: 10.1084/jem.20140508. Epub 2015 Jan 19. J Exp Med. 2015. PMID: 25601651 Free PMC article. - Profiling the immune response to Mycobacterium tuberculosis Beijing family infection: a perspective from the transcriptome.
María Irene CC, Juan Germán RC, Gamaliel LL, Dulce Adriana ME, Estela Isabel B, Brenda Nohemí MC, Payan Jorge B, Zyanya Lucía ZB, Myriam BDV, Fernanda CG, Adrian OL, Martha Isabel M, Rogelio HP. María Irene CC, et al. Virulence. 2021 Dec;12(1):1689-1704. doi: 10.1080/21505594.2021.1936432. Virulence. 2021. PMID: 34228582 Free PMC article. - F-actin and myosin II accelerate catecholamine release from chromaffin granules.
Berberian K, Torres AJ, Fang Q, Kisler K, Lindau M. Berberian K, et al. J Neurosci. 2009 Jan 21;29(3):863-70. doi: 10.1523/JNEUROSCI.2818-08.2009. J Neurosci. 2009. PMID: 19158310 Free PMC article.
References
- Cohen MS. Molecular events in the activation of human neutrophils for microbial killing. Clin. Infect. Dis. 1994;18 Suppl 2:S170–S179. - PubMed
- Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L419–L422. - PubMed
- Liu Y, et al. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J. Immunol. 2004;172:7–13. - PubMed
- Simpson SQ, Casey LC. Role of tumor necrosis factor in sepsis and acute lung injury. Crit. Care Clin. 1989;5:27–47. - PubMed
- Crockett-Torabi E, Ward PA. The role of leukocytes in tissue injury. Eur. J. Anaesthesiol. 1996;13:235–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous