Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson's disease - PubMed (original) (raw)
Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson's disease
Ruben K Dagda et al. Autophagy. 2008 Aug.
Abstract
Degenerating neurons of Parkinson's disease (PD) patient brains exhibit granules of phosphorylated extracellular signal-regulated protein kinase 1/2 (ERK1/2) that localize to autophagocytosed mitochondria. Here we show that 6-hydroxydopamine (6-OHDA) elicits activity-related localization of ERK1/2 in mitochondria of SH-SY5Y cells, and these events coincide with induction of autophagy and precede mitochondrial degradation. Transient transfection of wildtype (WT) ERK2 or constitutively active MAPK/ERK Kinase 2 (MEK2-CA) was sufficient to induce mitophagy to a degree comparable with that elicited by 6-OHDA, while constitutively active ERK2 (ERK2-CA) had a greater effect. We developed green fluorescent protein (GFP) fusion constructs of WT, CA, and kinase-deficient (KD) ERK2 to study the role of ERK2 localization in regulating mitophagy and cell death. Under basal conditions, cells transfected with GFP-ERK2-WT or GFP-ERK2-CA, but not GFP-ERK2-KD, displayed discrete cytoplasmic ERK2 granules of which a significant fraction colocalized with mitochondria and markers of autophagolysosomal maturation. The colocalizing GFP-ERK2/mitochondria granules are further increased by 6-OHDA and undergo autophagic degradation, as bafilomycin-A, an inhibitor of autolysosomal degradation, robustly increased their detection. Interestingly, increasing ERK2-WT or ERK2-CA expression was sufficient to promote comparable levels of macroautophagy as assessed by analysis of the autophagy marker microtubule-associated protein 1 light chain 3 (LC3). In contrast, the level of mitophagy was more tightly correlated with ERK activity levels, potentially explained by the greater localization of ERK2-CA to mitochondria compared to ERK2-WT. These data indicate that mitochondrial localization of ERK2 activity is sufficient to recapitulate the effects of 6-OHDA on mitophagy and autophagic cell death.
Figures
Figure 1
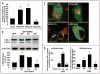
6-OHDA induces autophagy and ERK phosphorylation. Representative electron micrographs of cells treated with vehicle control (A) or 6-OHDA (B) for 4 hrs. Note that 6-OHDA increases the number of AVs and lysosomes (ly) and reduces mitochondria (m) content, while the integrity of the nucleus (n) is unaffected. Inset demonstrates an early AV containing distinct cytoplasmic material (scale bars: 1 μm). (C) Representative cells stably expressing GFP-LC3 in the presence or absence of 6-OHDA. The green channel was extracted to grayscale and inverted. (D) Effects of 6-OHDA treatment on GFP-LC3 puncta number (left bar graph) and size (right bar graph) were determined by using the NIH Image J macro described in Methods. For comparative purposes, the dotted line indicates the average number and size of GFP-LC3 puncta that accumulate due to bafilomycin-A treatment. Bar graphs show compiled means ± s.e.m. of at least 3 independent experiments (ø:p < 0.05 vs. untreated, ⊕:p < 0.001 vs. 6-OHDA). (E) SH-SY5Y cells were treated with 6-OHDA or with vehicle for the indicated time points. Following treatment with toxin, cells were immunoblotted for total and phospho-ERK1/2 and for LC3. (F) Quantification of the average number of GFP-LC3 puncta of transiently transfected cells co-treated with 6-OHDA for 4 h with or without the MEK inhibitor U0126. Bar graph shows means ± s.e.m. (30–40 cells analyzed per condition) and is representative of at least 3 independent experiments (ø: p < 0.0001 vs. basal, ⊗: p < 0.001 vs. 6-OHDA). Note that inhibition of MEK with U0126 suppresses 6-OHDA induction of GFP-LC3 puncta but has little effect on baseline autophagy.
Figure 2
6-OHDA promotes mitochondrial ERK1/2 phosphorylation and mitophagy. (A) Representative Western blot showing p-ERK1/2 in cytosolic (C) and in mitochondrial fractions (M) of SH-SY5Y cells treated with 6-OHDA for 4 hrs. compared to vehicle treated cells (V). (B) Representative confocal images of MitoTracker Red-stained (MTR) SH-SY5Y cells stably expressing GFP-LC3, treated with vehicle control or with 6-OHDA for 4 hrs. The insets, which have been enlarged by 50%, show the merged and separate channels for GFP-LC3 (1) and MTR (2) from a second 6-OHDA treated cell. (C) Quantification of the percent of GFP-LC3 puncta colocalizing with mitochondria. Bar graph shows means ± s.e.m. (25–30 cells analyzed per condition) and is representative of at least 3 independent experiments ø:p < 0.005 vs. vehicle ⊕:p < 0.005 vs. 6-OHDA). (D) Representative epifluorescence images of cells treated with vehicle control or with 4 hours of 6-OHDA, then immunolabeled using an antibody specific for human mitochondrial antigen of 60 kDa (MITO-P60). Note that 6-OHDA induces mitochondrial fragmentation. Scale bars: 20 μm. (E) SH-SY5Y cells were treated with 6-OHDA or with vehicle control for different time points. Cells were analyzed for total protein levels of mitochondria by immunoblotting for mitochondrial human antigen of 110 kDa (MITO-P110) and reprobed for β-actin as a loading control. (F) SH-SY5Y cells were treated with vehicle or 6-OHDA in the absence and presence of bafilomycin-A, and analyzed by Western blot for levels of the 60 kDa mitochondrial membrane protein (p60), and the matrix protein pyruvate dehydrogenase (PDH). (G, left) SH-SY5Y cells transiently transfected with GFP were treated with 6-OHDA for 5 h in the presence or absence of balilomycin-A. Cells were fixed, and immunostained for mitochondrial human antigen of 60 kDa. The effects of 6-OHDA on mitochondrial content was analyzed by measuring the percent cellular area occupied by mitochondria in GFP transfected cells using a custom made NIH Image J algorithm. Data was normalized to basal mitochondria content exhibited by non-treated cells. The representative bar graph shows means ± s.e.m. (22–50 cells analyzed per condition) (ø:p < 0.0001 vs. untreated, ⊕:p < 0.005 vs. 6-OHDA). (G, right) After 3 days, cells co-transfected with GFP and with siRNA targeting human Atg7 or LC3 were treated with 6-OHDA for 5 h and analyzed using Image J as described above. Data was normalized to basal mitochondria content exhibited by cells treated with each respective siRNA and not receiving toxin. The representative bar graph shows means ± s.e.m. (22–50 cells analyzed per condition) (ø:p < 0.0005 vs. untreated, ⊕:p < 0.0005 vs. scrambled siRNA with 6-OHDA, ⊗:p < 0.05 vs. scrambled siRNA with 6-OHDA).
Figure 3
Activation of ERK2 is sufficient to promote macroautophagy and mitophagy. (A) Quantification of the average number of GFP-LC3 puncta per cell in SH-SY5Y cells transiently co-transfected with GFP-LC3 and either vector, wild-type ERK2, constitutively active ERK2 (ERK2-CA), or kinase deficient ERK2 (ERK2-KD). ⊘:p < 0.005 vs. Vector.+:p < 0.0001 vs. ERK2-WT). The bar graph shows means ± s.e.m. (25–30 cells analyzed per condition) and is representative of >3 independent experiments. Supplementary Figure S5C and D shows that pharmacologic inhibition of MEK inhibits ERK2-WT-induced GFP-LC3 puncta. (B) Representative LC3 Western blot of SH-SY5Y cells transfected with GFP control or the indicated N-terminal GFP fusion constructs of ERK2 and re-probed for β-actin. The bar graph demonstrates LC3-II/β-actin ratios for three replicates of each transfection condition analyzed on the same Western blot (ø:p < 0.014 vs GFP, ⊕:p < 0.05 vs.GFP-ERK2-WT). (C) Representative confocal microscopy images of cells transiently co-expressing GFP-LC3 and vector, wild-type or the indicated mutant plasmids of ERK2. To image mitochondria, cells were loaded with MitoTracker Red (MTR) dye for 30 minutes prior to imaging by confocal microscopy (scale bar: 20 μm). (D, left) Intracellular ERK activities measured using the Elk1 trans-Reporting System in SH-SY5Y cells expressing different forms of ERK2. The Elk-1 luciferase reporter plasmid was co-transfected with the indicated ERK2 plasmids and activity was measured 48 hrs. following transfection. Bar graph shows means ± s.e.m of the fold increase relative to vector control (4–8 wells per transfection condition) and is representative of 3 independent experiments. (⊗:p < 0.0001 vs Vector, ⊕:p < 0.0001 vs ERK2-WT, ø:p < 0.009 vs. ERK2-WT). (D, right) Representative bar graph showing the percent of GFP-LC3 puncta colocalizing with mitochondria in cells expressing the indicated plasmids in the presence or absence of 6-OHDA. Bar graph show means ± s.e.m. (25–30 cells analyzed per condition) and are representative of at least 3 independent experiments (⊗:p < 0.003 vs. vector, ⊕:p < 0.0005 vs ERK2-WT, ø:p < 0.01 vs. ERK2-WT). Supplementary Figure S3 shows that transient expression of active ERK or MEK elevates mitophagy to levels similar to 6-OHDA as measured by GFP-LC3/colocalization and loss of cellular mitochondrial content.
Figure 4
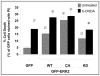
GFP-ERK2 exhibits activity dependent colocalization with mitochondria. (A) Quantification of the percentage of cells expressing GFP-ERK2-WT demonstrating the specified subcellular distribution of ERK2 for the indicated time points of 6-OHDA treatment. Note that 6-OHDA induces a time-dependent redistribution of GFP-ERK2-WT from the nucleus to the cytosol. The distribution bar graphs are representative of at least 3 independent experiments. (B) Representative confocal images of cells transiently expressing the indicated N-terminal GFP fusion constructs of ERK2 treated with vehicle control (left) or with 6-OHDA for 4 hrs. in the presence (right) or absence (middle) of bafilomycin-A. Mitochondria were imaged by loading cells with MitoTracker Red dye 30 min prior to imaging with the confocal microscope (scale bar: 20 μm). For some experiments mitochondria were visualized by co-transfecting cells with mito-RFP and GFP-ERK2 constructs, yielding similar colocalization results (not shown). White arrows point to ERK2-GFP puncta colocalizing with mitochondria. Note that 6-OHDA (top middle), but not vehicle control (top left), induces mitochondrial fragmentation and loss. Expression of ERK2-KD reduced mitochondrial loss, but not fragmentation (bottom middle). Supplementary Figure S1B and C shows that 6-OHDA does not induce any increase in triton-insoluble GFP or GFP-ERK2, and that comparable levels of GFP-ERK-WT and -CA are solubilized by triton X-100 versus SDS sample buffer, indicating that there is no aggregation occurring. (C) Representative Western blot of cells transfected with GFP or the indicated N-terminal GFP fusion constructs of ERK2 and immunoblotted for GFP and for total ERK1/2. Arrows heads point to immunoreactive bands corresponding to GFP-ERK2 constructs containing a predicted molecular weight of 73 kDa. Transfection efficiencies of all four GFP containing plasmids were ~25% across all experiments (Suppl. Fig. S1A). (D) Quantification of the average number of GFP-ERK2 granules per cell in untreated or 6-OHDA treated SH-SY5Y cells transiently transfected with GFP as a control or the indicated GFP fusion constructs of ERK2. Summary bar graph means ± s.e.m. of n = 3–7 experiments with 25–30 cells each (ø:p < 0.05 vs. GFP untreated, ⊕:p < 0.01 vs. untreated GFP-ERK2-WT, ⊗:p < 0.05 vs. GFP-ERK2-WT treated with 6-OHDA). (E) Summary quantification of the percent of GFP-ERK2 granules showing mitochondrial colocalization in the presence or absence of 6-OHDA (means ± s.e.m. of n = 3–7 experiments with 25–30 cells each; ø:p < 0.05 vs.GFP, ⊕:p < 0.005 vs untreated GFP-ERK2-WT, Φ:p < 0.05 vs. untreated GFP-ERK2-WT). Supplementary Figure S4 shows that the different plasmids show the same responses to MPP+, and Supplementary Figure S5A and B show that MEK activation of ERK2-WT is required for increase in number and mitochondrial colocalization of GFP-ERK2-WT granules. (F) Summary quantification of the percent of GFP-ERK2 granules colocalizing with mitochondria in the presence or absence of bafilomycin-A (means ± s.e.m. of n = 3–7 experiments with 25–30 cells each; ø:p < 0.02 vs. untreated GFP, ⊕:p < 0.01 vs. untreated GFP-ERK2-WT, ⊗:p < 0.05 vs. GFP-ERK2-WT with bafilomycin-A). Supplementary Figure S2 shows that bafilomycin-A also significantly increases numbers and mitochondrial colocalization of GFP-ERK2 granules in the presence of 6-OHDA.
Figure 5
GFP-ERK2 granules colocalize with autophagosomes and lysosomes. (A) Representative confocal sections of 6-OHDA treated cells transiently co-expressing GFP vector or GFP-ERK2 and an N-terminal RFP fusion of LC3 (Cherry-LC3), or (B) co-expressing an N-terminal RFP fusion of ERK2 (RFP-ERK2) and GFP-LC3, or (C) expressing RFP-ERK2 and stained for endogenous LC3 by immunofluorescence with blue nuclear counterstain (scale bar: 20 μm). Arrows point to LC3 puncta colocalizing with ERK2 granules. (D) Representative confocal sections of LysoTracker Red (LTR) stained cells expressing with GFP vector control or the indicated N-terminal GFP fusion constructs of ERK2 (scale bar: 20 μm). Arrows point to GFP-ERK2 granules colocalizing with lysosomes (E) Summary quantification of the percent of GFP-ERK2 granules colocalizing with lysosomes per cell (means ± s.e.m. of n = 3–5 experiments with 25–30 cells each; ⊕:p < 0.05 vs GFP, ø: p < 0.05 vs GFP-ERK2-WT. (F) Summary quantification of the average number of lysosomes per cell (means ± s.e.m. of n = 3–5 experiments with 25–30 cells each; ø:p < 0.05 vs. GFP, ⊗:p < 0.05 vs. GFP-ERK2-WT).
Figure 6
Autophagic cell stress induced by ERK2 expression promotes basal and 6-OHDA induced cell death. Cells transiently expressing GFP as a control or the indicated N-terminal GFP fusion constructs of ERK2 were analyzed for cell death by quantifying the percentage of GFP positive cells permeable to propidium iodide (PI) per epifluorescence micrograph field. The representative bar graph shows means ± s.e.m. (150–200 cells analyzed per condition collected from 7–10 random microscopic fields) and is representative of at least 3 independent experiments (ø:p < 0.005 vs. GFP, ⊗:p < 0.001 vs untreated GFP-ERK2-WT, ⊕:p < 0.05 vs. GFP with 6-OHDA, Φ:p < 0.05 vs. GFP-ERK2-WT with 6-OHDA).
Figure 7
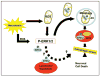
Schematic depicting a proposed role for ERK2 in mediating autophagic cell stress. The PD toxin 6-OHDA induces cytosolic and mitochondrial ROS, inducing robust phosphorylation of ERK2 and the formation of granules of ERK2 that colocalize with mitochondria, AVs and lysosomes (red ovals). Persistent activation of ERK2 induced by oxidative stress or by activating mutations promotes autophagy and mitophagy leading to autophagic cell stress and neurodegeneration. Although persistent ERK2 activation by toxin or experimental mutations induces lysosomal colocalization of ERK2 granules and lysosomal expansion, more evidence is needed to determine whether ERK2 might also be directly targeted to lysosomes and what role this signaling may play (hatched arrow).
Similar articles
- Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways.
Hirota Y, Yamashita S, Kurihara Y, Jin X, Aihara M, Saigusa T, Kang D, Kanki T. Hirota Y, et al. Autophagy. 2015;11(2):332-43. doi: 10.1080/15548627.2015.1023047. Autophagy. 2015. PMID: 25831013 Free PMC article. - The Degradation of TMEM166 by Autophagy Promotes AMPK Activation to Protect SH-SY5Y Cells Exposed to MPP.
Liao Z, Gong Z, Wang Z, Yang W, Liu W, Hou L, Liu X, Hua J, Wang B, Li N. Liao Z, et al. Cells. 2022 Aug 30;11(17):2706. doi: 10.3390/cells11172706. Cells. 2022. PMID: 36078115 Free PMC article. - Promotion of autophagy at the maturation step by IL-6 is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity.
Li XZ, Sui CY, Chen Q, Chen XP, Zhang H, Zhou XP. Li XZ, et al. Mol Cell Biochem. 2013 Aug;380(1-2):219-27. doi: 10.1007/s11010-013-1676-9. Epub 2013 May 16. Mol Cell Biochem. 2013. PMID: 23677697 - Mitochondrial function and autophagy: integrating proteotoxic, redox, and metabolic stress in Parkinson's disease.
Zhang J, Culp ML, Craver JG, Darley-Usmar V. Zhang J, et al. J Neurochem. 2018 Mar;144(6):691-709. doi: 10.1111/jnc.14308. Epub 2018 Feb 14. J Neurochem. 2018. PMID: 29341130 Free PMC article. Review. - The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition.
Meloche S, Pouysségur J. Meloche S, et al. Oncogene. 2007 May 14;26(22):3227-39. doi: 10.1038/sj.onc.1210414. Oncogene. 2007. PMID: 17496918 Review.
Cited by
- Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways.
Hirota Y, Yamashita S, Kurihara Y, Jin X, Aihara M, Saigusa T, Kang D, Kanki T. Hirota Y, et al. Autophagy. 2015;11(2):332-43. doi: 10.1080/15548627.2015.1023047. Autophagy. 2015. PMID: 25831013 Free PMC article. - Prognostic relevance of autophagy markers LC3B and p62 in esophageal adenocarcinomas.
Adams O, Dislich B, Berezowska S, Schläfli AM, Seiler CA, Kröll D, Tschan MP, Langer R. Adams O, et al. Oncotarget. 2016 Jun 28;7(26):39241-39255. doi: 10.18632/oncotarget.9649. Oncotarget. 2016. PMID: 27250034 Free PMC article. - Tetraspanin-induced death of myeloma cell lines is autophagic and involves increased UPR signalling.
Zismanov V, Lishner M, Tartakover-Matalon S, Radnay J, Shapiro H, Drucker L. Zismanov V, et al. Br J Cancer. 2009 Oct 20;101(8):1402-9. doi: 10.1038/sj.bjc.6605291. Epub 2009 Sep 15. Br J Cancer. 2009. PMID: 19755988 Free PMC article. - Development and validation of a screening assay for the evaluation of putative neuroprotective agents in the treatment of Parkinson's disease.
Yong-Kee CJ, Salomonczyk D, Nash JE. Yong-Kee CJ, et al. Neurotox Res. 2011 May;19(4):519-26. doi: 10.1007/s12640-010-9174-2. Epub 2010 Apr 2. Neurotox Res. 2011. PMID: 20361292 - The Tyrosine Phosphatase hPTPRβ Controls the Early Signals and Dopaminergic Cells Viability via the P2X7 Receptor.
Bernal FL, Luque Montoro M, Arrazola Sastre A, Lacerda HM, Zugaza JL. Bernal FL, et al. Int J Mol Sci. 2021 Nov 29;22(23):12936. doi: 10.3390/ijms222312936. Int J Mol Sci. 2021. PMID: 34884741 Free PMC article.
References
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–22. - PubMed
- Ventruti A, Cuervo AM. Autophagy and neurodegeneration. Curr Neurol Neurosci Rep. 2007;7:443–51. - PubMed
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 NS007495/NS/NINDS NIH HHS/United States
- DC009120/DC/NIDCD NIH HHS/United States
- K18 DC009120/DC/NIDCD NIH HHS/United States
- F32 AG030821/AG/NIA NIH HHS/United States
- R01 AG026389/AG/NIA NIH HHS/United States
- R01 AG026389-02/AG/NIA NIH HHS/United States
- AG026389/AG/NIA NIH HHS/United States
- F32 AG030821-01/AG/NIA NIH HHS/United States
- R01 AG026389-01A2/AG/NIA NIH HHS/United States
- K18 DC009120-01/DC/NIDCD NIH HHS/United States
- T32 NS07495/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous