Histoplasma capsulatum yeast phase-specific protein Yps3p induces Toll-like receptor 2 signaling - PubMed (original) (raw)
Histoplasma capsulatum yeast phase-specific protein Yps3p induces Toll-like receptor 2 signaling
Rajagopal N Aravalli et al. J Neuroinflammation. 2008.
Abstract
Histoplasma capsulatum is a common cause of fungal infection in certain geographic areas, and although most infections are asymptomatic, it is capable of causing histoplasmosis, a disseminated, life-threatening disease, especially in immunocompromised individuals. A deeper understanding of this host-pathogen interaction is needed to develop novel therapeutic strategies to counter lethal infection. Although several lines of evidence suggest that this fungus is neurotropic in HIV patients, little is known about the immunobiology of Histoplasma infection in the central nervous system [CNS]. The goal of the present study was to understand the innate neuroimmune mechanisms that recognize H. capsulatum during the initial stages of infection. Using a 293T stable cell line expressing murine Toll-like receptor 2 [TLR2], we show here that TLR2 recognizes H. capsulatum cell wall protein Yps3p and induces the activation of NF-kappaB. In further experiments, we tested the ability of Yps3p to induce signaling from TLR2 in primary microglial cells, the resident brain macrophages of the CNS. Our data show that H. capsulatum Yps3p induced TLR2 signaling in wild-type microglia, but not in microglia isolated from TLR2 KO mice, confirming that Yps3p is a ligand for TLR2. Furthermore, Yps3p-induced TLR2 signaling was suppressed by vaccinia virus-encoded TLR inhibitors. This is the first demonstration of a fungal protein serving as a TLR ligand and mediating signaling in primary brain cells.
Figures
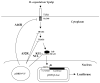
Figure 1
Experimental design. Induction of TLR signaling by H. capsulatum Yps3p protein leads to the activation of IRAK2 and NF-κB. NF-κB is then released from its inhibitor IκB and translocates into the nucleus, where it binds to one of five NF-κB binding sites on the pNiFty2-Luc plasmid, and subsequently activates transcription to produce luciferase. Vaccinia virus [VV] protein A46R targets multiple TLR adaptors including MyD88, and A52R associates with IRAK-1 and TRAF6 to disrupt downstream signaling. VV N1L and K1L proteins prevent the release of NF-κB from IκB. Expression of these proteins from their corresponding pORF5-VV plasmid leads to the inhibition of NF-κB activation and decreased luciferase expression from pNiFty2-Luc.
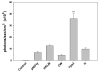
Figure 2
Identification of Yps3p as a TLR2 ligand in 293T cells. pNiFty2-Luc transfected 293T-mTLR2 cells were treated with H. capsulatum cell wall/membrane fraction [CW], recombinant Yps3p [Yps3p], and recombinant protein H [H]. Untreated cells were used as a control for background luciferase expression [pNIFty] and heat-killed Listeria monocytogenes [HKLM] was added to cells as a positive control for TLR2 signaling. Data are presented as mean ± SD of triplicate samples and are representative of three independent experiments. Statistical analysis was performed by student's t test. *P < 0.05; **P < 0.01.
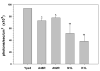
Figure 3
Inhibition of TLR2-mediated, Yps3p-induced NF-κB activation in 293T-mTLR2 cells by VV proteins. Plasmids carrying the open-reading frames of A46R, A52R, K1L, or N1L were co-transfected along with pNiFty2-Luc, cells were incubated overnight at 37°C and treated with Yps3p for 5 h. Cells were then harvested and the amount of luciferase produced was quantified using bright glow substrate. Data are presented as mean ± SD of triplicate samples and are representative of three independent experiments. Statistical analysis was performed by student's t test. *P < 0.05; **P < 0.01.
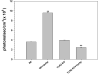
Figure 4
Activation of NF-κB in primary murine microglia following treatment with Yps3p. Purified microglial cells [1 × 106] from wild-type and TLR2 knockout mice were transiently transfected with pNiFty2-Luc plasmid by electroporation using the mouse macrophage nucleofector kit. The cells were incubated overnight at 37°C and 1.5 μg Yps3p was added to the cell culture medium. The microglia were harvested after 5 h and the amount of luciferase produced as a result of NF-κB activation was quantified using the bright glow substrate. Data are presented as mean ± SD of triplicate samples and are representative of three independent experiments. Statistical analysis was performed by student's t test. **P < 0.01.
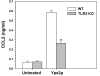
Figure 5
TLR2-mediated expression of CCL2 in response to Yps3p. ELISA assay was performed with purified microglial cells from wild-type and TLR2 knockout mice following exposure with 3 μg/ml Yps3p. Data are presented as relative induction of CCL2 and bars represent the mean ± SD of triplicate samples, which are representative of at least three independent experiments. Statistical analysis was performed by student's t test. **P < 0.01.
Similar articles
- Surface localization of the Yps3p protein of Histoplasma capsulatum.
Bohse ML, Woods JP. Bohse ML, et al. Eukaryot Cell. 2005 Apr;4(4):685-93. doi: 10.1128/EC.4.4.685-693.2005. Eukaryot Cell. 2005. PMID: 15821128 Free PMC article. - Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages.
Inglis DO, Berkes CA, Hocking Murray DR, Sil A. Inglis DO, et al. Infect Immun. 2010 Sep;78(9):3871-82. doi: 10.1128/IAI.00204-10. Epub 2010 Jul 6. Infect Immun. 2010. PMID: 20605974 Free PMC article. - Histoplasma capsulatum cell wall {beta}-glucan induces lipid body formation through CD18, TLR2, and dectin-1 receptors: correlation with leukotriene B4 generation and role in HIV-1 infection.
Sorgi CA, Secatto A, Fontanari C, Turato WM, Belangér C, de Medeiros AI, Kashima S, Marleau S, Covas DT, Bozza PT, Faccioli LH. Sorgi CA, et al. J Immunol. 2009 Apr 1;182(7):4025-35. doi: 10.4049/jimmunol.0801795. J Immunol. 2009. PMID: 19299700 - [Relevant aspects of the Hcp100 molecular marker of Histoplasma capsulatum and its potential therapeutic use in histoplasmosis].
González-González AE, Taylor ML, Curiel-Quesada E. González-González AE, et al. Rev Iberoam Micol. 2012 Jul-Sep;29(3):115-9. doi: 10.1016/j.riam.2011.09.001. Epub 2011 Oct 25. Rev Iberoam Micol. 2012. PMID: 22037114 Review. Spanish. - Histoplasma capsulatum causing sinusitis: a case report in French Guiana and review of the literature.
Nabet C, Belzunce C, Blanchet D, Abboud P, Djossou F, Carme B, Aznar C, Demar M. Nabet C, et al. BMC Infect Dis. 2018 Nov 26;18(1):595. doi: 10.1186/s12879-018-3499-5. BMC Infect Dis. 2018. PMID: 30477434 Free PMC article. Review.
Cited by
- Role of microglia in fungal infections of the central nervous system.
Koutsouras GW, Ramos RL, Martinez LR. Koutsouras GW, et al. Virulence. 2017 Aug 18;8(6):705-718. doi: 10.1080/21505594.2016.1261789. Epub 2016 Nov 18. Virulence. 2017. PMID: 27858519 Free PMC article. Review. - Antifungal Encapsulated into Ligand-Functionalized Nanoparticles with High Specificity for Macrophages.
Mejía SP, López D, Cano LE, Naranjo TW, Orozco J. Mejía SP, et al. Pharmaceutics. 2022 Sep 13;14(9):1932. doi: 10.3390/pharmaceutics14091932. Pharmaceutics. 2022. PMID: 36145686 Free PMC article. - Common virulence factors between Histoplasma and Paracoccidioides: Recognition of Hsp60 and Enolase by CR3 and plasmin receptors in host cells.
de Matos Silva S, Echeverri CR, Mendes-Giannini MJS, Fusco-Almeida AM, Gonzalez A. de Matos Silva S, et al. Curr Res Microb Sci. 2024 Jun 8;7:100246. doi: 10.1016/j.crmicr.2024.100246. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 39022313 Free PMC article. Review. - Revisiting old friends: Developments in understanding Histoplasma capsulatum pathogenesis.
Woods JP. Woods JP. J Microbiol. 2016 Mar;54(3):265-76. doi: 10.1007/s12275-016-6044-5. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920886 Review. - Histoplasmosis with Deep CNS Involvement: Case Presentation with Discussion and Literature Review.
Hariri OR, Minasian T, Quadri SA, Dyurgerova A, Farr S, Miulli DE, Siddiqi J. Hariri OR, et al. J Neurol Surg Rep. 2015 Jul;76(1):e167-72. doi: 10.1055/s-0035-1554932. Epub 2015 Jun 26. J Neurol Surg Rep. 2015. PMID: 26251798 Free PMC article.
References
- Vos MJ, Debets-Ossenkopp YJ, Claessen FA, Hazenberg GJ, Heimans JJ. Cerebellar and medullar histoplasmosis. Neurology. 2000;54:1441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials