Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal-regulated kinase - PubMed (original) (raw)
Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal-regulated kinase
Charles R Gerfen et al. J Neurosci. 2008.
Abstract
Dopamine receptor signaling exhibits prominent plasticity that is important for the pathogenesis of both addictive and movement disorders. Psychoactive stimulants that activate the dopamine D(1) receptor (Drd1a) induce the rapid phosphorylation and activation of extracellular signal-regulated kinase 1/2 (ERK1/2) in neurons of the nucleus accumbens and ventral striatum. This response is known to be dependent on the phosphatase inhibitor dopamine- and cAMP-regulated phosphoprotein-32 (DARPP-32) and appears critical for the sensitization of Drd1a responses that contributes to addiction. Loss of dopamine input to the striatum, as in models of Parkinson's disease (PD), also results in a sensitization of responses to dopamine agonists that is manifest by increased activation of ERK1/2 in the dorsal striatum. Here, we test whether DARPP-32 is required for sensitization of Drd1a responses in a PD model. In the normal dorsal striatum, there is minimal Drd1a-mediated activation of ERK1/2; however, in the PD model there is robust Drd1a-mediated activation of ERK1/2. In both wild-type and DARPP-32 knock-out mice, Drd1a robustly induces pERK1/2 throughout the dopamine-depleted striatum. These findings indicate that Drd1a sensitization relevant for PD occurs by a novel mechanism that does not require DARPP-32.
Figures
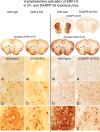
Figure 1.
Psychostimulant-induced activation of ERK1/2 in Drd1a knock-out and DARPP-32 knock-out mice. Effects of
d
-amphetamine treatment (10 mg/kg) are compared between wild-type and Drd1a KO mice and between wild-type and DARPP-32 KO mice, in which DARPP-32-IR in the striatum is absent. Activation of ERK1/2 is indicated in coronal brain sections by neurons displaying immunoreactive phosphorylated ERK1/2 (phospho-ERK1/2-IR). In the dorsolateral striatum (a) and in the dorsomedial striatum (b), the numbers of scattered neurons with
d
-amphetamine-induced immunoreactive phospho-ERK1/2 is similar in the wild-type and Drd1a KO and DARPP-32 KO animals. In the shell of the nucleus accumbens (c), there are numerous
d
-amphetamine-induced phospho-ERK1/2-immunoreactive neurons in the wild-type mice, whereas in both the Drd1a KO and DARPP-32 KO mice, the numbers of such neurons is markedly reduced. These data indicate that psychostimulant activation of ERK1/2 mediated by Drd1a and DARPP-32 is restricted to the nucleus accumbens.
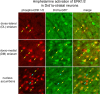
Figure 2.
d
-Amphetamine activation of ERK1/2 in Drd1a-expressing striatal neurons. Drd1a-BAC-EGFP transgenic mice were treated with
d
-amphetamine (10 mg/kg), and brain sections containing the striatum were processed for colocalization of phospho-ERK1/2-IR (red fluorochrome) and GFP-IR (green fluorochrome). A 100 μm2 area of the dorsolateral (DL), dorsomedial (DM), and nucleus accumbens is shown. The numbers of phospho-ERK1/2-immunoreactive neurons vary from few in the DL to many in the nucleus accumbens. In each region, nearly all phospho-ERK1/2-immunoreactive neurons colocalize with GFP (yellow arrows), which is produced in Drd1a striatal neurons. There are some rare phospho-ERK1/2-immunoreactive neurons that are GFP-IR negative (blue arrows) clearly evident as dark lacunas in the background of GFP fluorescence. These data indicate nearly all striatal neurons in which ERK1/2 is activated after
d
-amphetamine treatment express the Drd1a receptor.
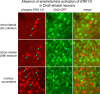
Figure 3.
d
-Amphetamine activates ERK1/2 in few Drd2-expressing striatal neurons. Drd2-BAC-EGFP transgenic mice were treated with
d
-amphetamine (10 mg/kg), and brain sections containing the striatum were processed for colocalization of phospho-ERK1/2-IR (red fluorochrome) and GFP-IR (green fluorochrome). A 100 μm2 area of the dorsolateral (DL), dorsomedial (DM), and nucleus accumbens is shown. The numbers of phospho-ERK1/2-immunoreactive neurons vary from few in the DL to many in the nucleus accumbens. In each region, nearly all phospho-ERK1/2-immunoreactive neurons do not express GFP, which is produced in Drd2-striatal neurons. These data indicate that very few striatal neurons in which ERK1/2 is activated after
d
-amphetamine treatment express the Drd2 receptor.
Figure 4.
Drd1a-agonist or
l
-DOPA activation of ERK1/2 in the dopamine (DA)-depleted striatum does not involve DARPP-32. Comparison of coronal brain sections at the level of the rostral striatum from wild-type and DARPP-32 KO mice, with unilateral lesions of the nigrostriatal dopamine system and treated with a Drd1a agonist (SKF-81297; 5 mg/kg; 1 d) or
l
-DOPA (20 mg/kg with 12 mg/kg benserazide; 10 d). DARPP-32-IR labels neurons in the striatum in wild-type mice, which are unlabeled in DARPP-32 KO mice. Unilateral lesion of the nigrostriatal dopamine pathway in these animals is shown by the absence of TH-IR in the axonal terminals in the right striatum. Activation of ERK1/2 in response to either Drd1a agonist treatment (left images) or
l
-DOPA treatment (right images) is demonstrated by phospho-ERK1/2-IR throughout the dopamine-depleted striatum. High-power images from the dorsolateral striatum (inset boxes; 100 μm wide) show few to no phospho-ERK1/2-immunoreactive neurons in the DA-intact striatum. In contrast, there are numerous phospho-ERK1/2-immunoreactive neurons in the DA-depleted striatum in both the wild-type and DARPP-32 KO animals.
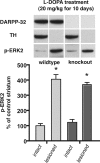
Figure 5.
l
-DOPA activation of ERK2 in the dopamine-depleted striatum is not significantly reduced in DARPP-32 knock-out mice. Western immunoblot data from wild-type (n = 7) and DARPP-32 knock-out (n = 5) animals with unilateral 6-OHDA lesions of the nigrostriatal dopamine pathway treated for 10 d with
l
-DOPA (20 mg/kg with 12 mg/kg benserazide). Animals were killed 30 min after the last injection and the dissected striatum were processed by Western blotting to determine levels of immunoreactivity for DARPP-32, TH, and phosphorylated ERK2 (pERK2). There is robust activation of pERK2 in the dopamine-depleted compared with the intact striatum of both wild-type and DARPP32 KO animals (*p < 0.05). However, there is no significant difference in the activation of pERK2 in the dopamine-lesioned striatum comparing wild-type and DARPP-32 KO animals (percentage above intact control: wild type, 406%; KO, 371%; _p_ > 0.05).
Similar articles
- Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA. Bertran-Gonzalez J, et al. J Neurosci. 2008 May 28;28(22):5671-85. doi: 10.1523/JNEUROSCI.1039-08.2008. J Neurosci. 2008. PMID: 18509028 Free PMC article. - Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol.
Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Valjent E, et al. Neuropsychopharmacology. 2010 Jan;35(2):401-15. doi: 10.1038/npp.2009.143. Neuropsychopharmacology. 2010. PMID: 19759531 Free PMC article. - Quantitative changes in Galphaolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants.
Corvol JC, Valjent E, Pascoli V, Robin A, Stipanovich A, Luedtke RR, Belluscio L, Girault JA, Hervé D. Corvol JC, et al. Neuropsychopharmacology. 2007 May;32(5):1109-21. doi: 10.1038/sj.npp.1301230. Epub 2006 Oct 25. Neuropsychopharmacology. 2007. PMID: 17063155 - Extracellular signal-regulated protein kinases 1 and 2 activation by addictive drugs: a signal toward pathological adaptation.
Pascoli V, Cahill E, Bellivier F, Caboche J, Vanhoutte P. Pascoli V, et al. Biol Psychiatry. 2014 Dec 15;76(12):917-26. doi: 10.1016/j.biopsych.2014.04.005. Epub 2014 Apr 18. Biol Psychiatry. 2014. PMID: 24844603 Review. - Effects of nicotine on DARPP-32 and CaMKII signaling relevant to addiction.
Lee AM, Picciotto MR. Lee AM, et al. Adv Pharmacol. 2021;90:89-115. doi: 10.1016/bs.apha.2020.09.002. Epub 2020 Oct 6. Adv Pharmacol. 2021. PMID: 33706940 Free PMC article. Review.
Cited by
- DARPP-32, Jack of All Trades… Master of Which?
Yger M, Girault JA. Yger M, et al. Front Behav Neurosci. 2011 Sep 8;5:56. doi: 10.3389/fnbeh.2011.00056. eCollection 2011. Front Behav Neurosci. 2011. PMID: 21927600 Free PMC article. - Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice.
Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Hervé D, Girault JA. Matamales M, et al. PLoS One. 2009;4(3):e4770. doi: 10.1371/journal.pone.0004770. Epub 2009 Mar 10. PLoS One. 2009. PMID: 19274089 Free PMC article. - Targeting β-arrestin2 in the treatment of L-DOPA-induced dyskinesia in Parkinson's disease.
Urs NM, Bido S, Peterson SM, Daigle TL, Bass CE, Gainetdinov RR, Bezard E, Caron MG. Urs NM, et al. Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2517-26. doi: 10.1073/pnas.1502740112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918399 Free PMC article. - Dopamine- and cAMP-regulated phosphoprotein of 32-kDa (DARPP-32)-dependent activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) signaling in experimental parkinsonism.
Santini E, Feyder M, Gangarossa G, Bateup HS, Greengard P, Fisone G. Santini E, et al. J Biol Chem. 2012 Aug 10;287(33):27806-12. doi: 10.1074/jbc.M112.388413. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753408 Free PMC article. - Dysregulated intracellular signaling in the striatum in a pathophysiologically grounded model of Tourette syndrome.
Rapanelli M, Frick LR, Pogorelov V, Ota KT, Abbasi E, Ohtsu H, Pittenger C. Rapanelli M, et al. Eur Neuropsychopharmacol. 2014 Dec;24(12):1896-906. doi: 10.1016/j.euroneuro.2014.10.007. Epub 2014 Nov 4. Eur Neuropsychopharmacol. 2014. PMID: 25464894 Free PMC article.
References
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, et al. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. - PubMed
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z99 MH999999/ImNIH/Intramural NIH HHS/United States
- R37 DA010309/DA/NIDA NIH HHS/United States
- DA11742/DA/NIDA NIH HHS/United States
- R01 DA011742-08/DA/NIDA NIH HHS/United States
- Z01 MH002497-18/ImNIH/Intramural NIH HHS/United States
- R01 DA011742/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous