Maintenance of tolerance by regulation of anti-myeloperoxidase B cells - PubMed (original) (raw)
Maintenance of tolerance by regulation of anti-myeloperoxidase B cells
Donna O Bunch et al. J Am Soc Nephrol. 2008 Sep.
Abstract
Anti-neutrophil cytoplasmic autoantibodies directed toward myeloperoxidase or proteinase 3 are detected in sera of patients with small vessel vasculitis and participate in the pathogenesis of this disease. Autoantibodies develop when self-reactive B cells escape the regulation that ensures self-tolerance. In this study, regulation of anti-myeloperoxidase B cells was examined in mice that express an anti-myeloperoxidase Vkappa1C-Jkappa5 light-chain transgene, which confers anti-myeloperoxidase specificity when combined with a variety of heavy chains. Vkappa1C-Jkappa5 transgenic mice have splenic anti-myeloperoxidase B cells but do not produce circulating anti-myeloperoxidase antibodies. Two groups of transgenic mice that differed by their relative dosage of the transgene were compared; high-copy mice had a mean relative transgene dosage of 1.92 compared with 1.02 in the low-copy mice. These mice exhibited a 90 and 60% decrease in mature follicular B cells, respectively. High-copy mice were characterized by a large population of anti-myeloperoxidase B cells, a preponderance of B-1 cells, and an increased percentage of apoptotic myeloperoxidase-binding B cells. Low-copy mice had similar changes in B cell phenotype with the exception of an expanded marginal zone population. B cells from low-copy mice but not high-copy mice produced anti-myeloperoxidase antibodies after stimulation with lipopolysaccharide. These results indicate that tolerance to myeloperoxidase is maintained by central and peripheral deletion and that some myeloperoxidase-binding B cells are positively selected into the marginal zone and B-1 B cell subsets. A defect in these regulatory pathways could result in autoimmune disease.
Figures
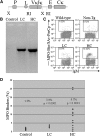
Figure 1.
MPO-binding B cells are found in spleens of Tg mice. (A) Diagram of the transgene construct. Locations of the endogenous Vκ21C promoter (P), Vκ21C leader sequence (L), rearranged Vκ1C/Jκ5 light chain (VκJκ), Cκ enhancer (E), and Cκ secretory exons (Cκ) are indicated. The EcoRI (RI) and XbaI (X) restriction sites used for subcloning are depicted. (B) Tg mice express the Vκ1C/Jκ5 light-chain transgene. Shown are PCR products from 1 ng of DNA from non-Tg control mice, LC mice, and HC mice. Both groups of Tg mice yield the Tg PCR product; non-Tg controls are negative. For identification of MPO-binding cells by FACS analysis, splenic cells were incubated with biotinylated MPO followed by streptavidin-peridinin chlorophyll protein. Cells positive for IgM and/or B220 were defined as B cells. (C) Representative histograms of a C57Bl/6 wild-type control mouse, a non-Tg littermate control mouse, an LC Tg mouse, and an HC Tg mouse that demonstrate the paucity of MPO-binding B cells in control mice compared with LC Tg mice and the distinct population of MPO binders found in HC Tg mice. (D) Percentage of MPO-binding splenic B cells is summarized. In contrast to the low level of MPO-binding B cells found in controls (n = 13), LC mice (n = 7) have significantly larger populations of MPO-binding B cells (P < 0.0001), and many of the HC mice (n = 14) have robust populations of MPO-binding B cells.
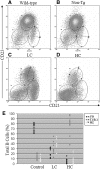
Figure 2.
Percentages of follicular, marginal zone, and T1/B-1 cells are altered in Vκ1C/Jκ5 Tg mice. (A through D) Representative CD21/CD23 histograms for a C57BL/6 wild-type control (A), a non-Tg littermate control (B), an LC mouse (C), and an HC mouse (D). The majority of B cells in control mice are mature follicular cells. LC mice have increased percentages of marginal zone and T1/B-1 cells, which parallel a decrease in the percentage of mature follicular cells. In HC mice, the majority of cells are T1/B-1 with few marginal zone or follicular cells. (E) The percentages of follicular, marginal zone, or T1/B-1 cells based on CD21/CD23 staining of splenic B cells from each type of mice are summarized. LC mice consistently have higher percentages of marginal zone and T1/B-1, whereas HC mice have mostly T1/B-1 cells.
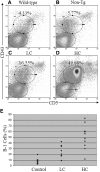
Figure 3.
Vκ1C/Jκ5 Tg mice have an increased percentage of B-1 cells. (A through D) Representative CD5/CD43 histograms for a B6 wild-type control (A), a non-Tg littermate control (B), an LC mouse (C), and an HC mouse (D). Percentages of B-1 cells are given in the corner of each histogram. Wild-type and non-Tg control mice have normal percentages of B-1 cells. The LC mice have a slight increase in the percentage of B-1 cells, whereas a dramatic increase in B-1 cells is observed in the HC mice. (E) Shown is a summary of the percentages of splenic B cells classified as B-1 (CD5+/CD43+) for mice in each group. Both LC and HC mice have increased percentages of B-1 cells compared with non-Tg control mice.
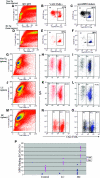
Figure 4.
MPO-binding B cells are increased in immature bone marrow cells of Vκ1C/Jκ5 Tg mice and T1/B1 cells in the spleen. (A, D, and G) Representative histograms for a non-Tg control (A), LC Tg mouse (D), and HC Tg mouse (G) depicting the proportion of pro-/pre-B (B220lo/IgMneg), immature B (B220lo/IgM+), and recirculating B (B220hi/IgM+) cells in the bone marrow. (B, E, and H) Histograms gated on R5 immature B cells show the percentage of B220lo MPO-binding B cells for a non-Tg control (B), an LC mouse (E), and an HC mouse (H). The percentage of immature MPO-binding B cells is increased in Tg mice. (C, F, and I) Representative histograms gated on IgM+/MPO+ cells show the percentage of MPO-binding follicular (CD21int/CD23+), marginal zone (CD21+/CD23neg), and T1/B-1 (CD21lo/CD23neg) for a non-Tg (C), an LC (F) and an HC (I) mouse.
Figure 5.
Apoptotic MPO-binding B cells are increased in Vκ1C/Jκ5 Tg mice. Representative histograms of cells from the bone marrow of a non-Tg (A through C) and an HC Tg (D through F) are presented. A white oval depicts the population based on forward versus side scatter that is considered apoptotic (A and D). Histograms in B and E confirm that the cells in the apoptotic gate based on forward versus side scatter are VAD-FMK+. Histograms in C and F demonstrate that 96 and 98% of the B220+/IgM+ MPO-binding B cells (shown in blue) in the apoptotic gate of non-Tg (C) and HC Tg (F) mice are VAD-FMK+. (G, J, and M) Representative forward and side scatter histograms for splenocytes from a B6 wild-type control (G), an LC Tg mouse (J), and an HC Tg mouse (M) are shown. The region referred to as the apoptotic cell gate is indicated by a white oval. The apoptotic status of cells in the apoptotic cell gate was confirmed by staining with VAD-FMK. IgM/VAD-FMK histograms for B220+ cells from the apoptotic gate are shown for a B6 wild-type control (H and I), an LC mouse (K and L), and an HC mouse (N and O). A majority of B cells in the apoptotic cell gate are VAD-FMK+ as depicted in H, K, and N; percentages shown are VAD-FMK+ cells. MPO binders (shown in blue in I, L, and O) are apoptotic and are found in greater abundance in Tg mice; percentages given are VAD-FMK+ MPO binders. Data demonstrating that MPO-binding B cells in the spleen are increased in the apoptotic cell gate is summarized in P. Shown are the percentages of MPO-binding B cells in the black oval normal lymphocyte gate (♦) and in the white oval apoptotic cell gate (▪) for each group of mice. Although there are more MPO binders in the apoptotic cell gate than in the normal lymphocyte gate in each group, the increased population of apoptotic cells that bind MPO is striking in LC mice (44.9%) and in HC mice (62.5%).
Similar articles
- Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern.
Li Y, Li H, Ni D, Weigert M. Li Y, et al. J Exp Med. 2002 Dec 16;196(12):1543-52. doi: 10.1084/jem.20021560. J Exp Med. 2002. PMID: 12486097 Free PMC article. - Restriction in V kappa gene use and antigen selection in anti-myeloperoxidase response in mice.
Jethwa HS, Clarke SH, Itoh-Lindstrom Y, Falk RJ, Jennette JC, Nachman PH. Jethwa HS, et al. J Immunol. 2000 Oct 1;165(7):3890-7. doi: 10.4049/jimmunol.165.7.3890. J Immunol. 2000. PMID: 11034396 - Persistence of functionally compromised anti-double-stranded DNA B cells in the periphery of non-autoimmune mice.
Roark JH, Bui A, Nguyen KA, Mandik L, Erikson J. Roark JH, et al. Int Immunol. 1997 Nov;9(11):1615-26. doi: 10.1093/intimm/9.11.1615. Int Immunol. 1997. PMID: 9418123 - Kappa editing rescues autoreactive B cells destined for deletion in mice transgenic for a dual specific anti-laminin Ig.
Brady GF, Congdon KL, Clark AG, Sackey FN, Rudolph EH, Radic MZ, Foster MH. Brady GF, et al. J Immunol. 2004 May 1;172(9):5313-21. doi: 10.4049/jimmunol.172.9.5313. J Immunol. 2004. PMID: 15100270 - T and B cell tolerance and responses to viral antigens in transgenic mice: implications for the pathogenesis of autoimmune versus immunopathological disease.
Zinkernagel RM, Pircher HP, Ohashi P, Oehen S, Odermatt B, Mak T, Arnheiter H, Bürki K, Hengartner H. Zinkernagel RM, et al. Immunol Rev. 1991 Aug;122:133-71. doi: 10.1111/j.1600-065x.1991.tb00601.x. Immunol Rev. 1991. PMID: 1937540 Review.
Cited by
- A glance into the future of anti-neutrophil cytoplasmic antibody-associated vasculitis.
Casal Moura M, Branco C, Martins-Martinho J, Ferraro JL, Berti A, Nogueira E, Ponte C. Casal Moura M, et al. Ther Adv Musculoskelet Dis. 2022 Nov 4;14:1759720X221125979. doi: 10.1177/1759720X221125979. eCollection 2022. Ther Adv Musculoskelet Dis. 2022. PMID: 36353270 Free PMC article. Review. - A Meta-Analysis of the Safety and Efficacy of Maintenance Therapies for Antineutrophil Cytoplasmic Antibody Small-Vessel Vasculitis.
Bellos I, Boletis I, Lionaki S. Bellos I, et al. Kidney Int Rep. 2022 Mar 2;7(5):1074-1083. doi: 10.1016/j.ekir.2022.02.020. eCollection 2022 May. Kidney Int Rep. 2022. PMID: 35570996 Free PMC article. - Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis.
Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R. Bontscho J, et al. J Am Soc Nephrol. 2011 Feb;22(2):336-48. doi: 10.1681/ASN.2010010034. Epub 2011 Jan 13. J Am Soc Nephrol. 2011. PMID: 21233415 Free PMC article. - Update on pathogenic mechanisms of systemic necrotizing vasculitis.
Danila MI, Bridges SL Jr. Danila MI, et al. Curr Rheumatol Rep. 2008 Dec;10(6):430-5. doi: 10.1007/s11926-008-0070-1. Curr Rheumatol Rep. 2008. PMID: 19007531 Review.
References
- Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med 337: 1512–1523, 1997 - PubMed
- Kettritz R, Choi M, Butt W, Rane M, Rolle S, Luft FC, Klein JB: Phosphatidylinositol 3-kinase controls antineutrophil cytoplasmic antibodies-induced respiratory burst in human neutrophils. J Am Soc Nephrol 13: 1740–1749, 2002 - PubMed
- Kobayashi K, Shibata T, Sugisaki T: Aggravation of rat nephrotoxic serum nephritis by anti-myeloperoxidase antibodies. Kidney Int 47: 454–463, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI043587/AI/NIAID NIH HHS/United States
- AI 43587/AI/NIAID NIH HHS/United States
- P01 DK58335/DK/NIDDK NIH HHS/United States
- P01 DK058335/DK/NIDDK NIH HHS/United States
- AI 29576/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials