An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer - PubMed (original) (raw)
. 2008 Aug 1;68(15):6084-91.
doi: 10.1158/0008-5472.CAN-07-6854.
Ana Maria Gonzalez-Angulo, Ana Lluch, Richard M Neve, Wen-Lin Kuo, Michael Davies, Mark Carey, Zhi Hu, Yinghui Guan, Aysegul Sahin, W Fraser Symmans, Lajos Pusztai, Laura K Nolden, Hugo Horlings, Katrien Berns, Mien-Chie Hung, Marc J van de Vijver, Vicente Valero, Joe W Gray, René Bernards, Gordon B Mills, Bryan T Hennessy
Affiliations
- PMID: 18676830
- PMCID: PMC2680495
- DOI: 10.1158/0008-5472.CAN-07-6854
An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer
Katherine Stemke-Hale et al. Cancer Res. 2008.
Abstract
Phosphatidylinositol 3-kinase (PI3K)/AKT pathway aberrations are common in cancer. By applying mass spectroscopy-based sequencing and reverse-phase protein arrays to 547 human breast cancers and 41 cell lines, we determined the subtype specificity and signaling effects of PIK3CA, AKT, and PTEN mutations and the effects of PIK3CA mutations on responsiveness to PI3K inhibition in vitro and on outcome after adjuvant tamoxifen. PIK3CA mutations were more common in hormone receptor-positive (34.5%) and HER2-positive (22.7%) than in basal-like tumors (8.3%). AKT1 (1.4%) and PTEN (2.3%) mutations were restricted to hormone receptor-positive cancers. Unlike AKT1 mutations that were absent from cell lines, PIK3CA (39%) and PTEN (20%) mutations were more common in cell lines than tumors, suggesting a selection for these but not AKT1 mutations during adaptation to culture. PIK3CA mutations did not have a significant effect on outcome after adjuvant tamoxifen therapy in 157 hormone receptor-positive breast cancer patients. PIK3CA mutations, in comparison with PTEN loss and AKT1 mutations, were associated with significantly less and inconsistent activation of AKT and of downstream PI3K/AKT signaling in tumors and cell lines. PTEN loss and PIK3CA mutation were frequently concordant, suggesting different contributions to pathophysiology. PTEN loss rendered cells significantly more sensitive to growth inhibition by the PI3K inhibitor LY294002 than did PIK3CA mutations. Thus, PI3K pathway aberrations likely play a distinct role in the pathogenesis of different breast cancer subtypes. The specific aberration present may have implications for the selection of PI3K-targeted therapies in hormone receptor-positive breast cancer.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
J.W. Gray: commercial research grants, GlaxoSmithKline, Cellgate, Affymetrix, and Cell Biosciences; consultant, Agendia, Cepheid, and Bristol-Myers Squibb. G.B. Mills: scientific/advisory committee member, Abbott Laboratories, Ambit Biosciences Corp., Lpath Therapeutics Inc., and Texas Institute for Genomic Medicine; consultant, GlaxoSmithKline, Semafore Pharmaceuticals Inc., and TAU Therapeutics; stock options, GLT, Inc.; royalty income, Upstate Biotechnology. The other authors disclosed no potential conflicts of interest.
Figures
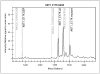
Figure 1
Detection of PIK3CA and AKT1 mutations. A mass spectrometry tracing of an AKT1 E17K-mutant SNP allele in a hormone receptor–positive breast cancer. wt, wild type.
Figure 2
Effects of PTEN loss and PIK3CA mutation on AKT activation in human breast tumors. PTEN and the two AKT phosphorylation sites (AKTp308 and AKTp473) were quantified using reverse-phase protein lysate array. The quantification data were then log transformed (base 2), mean centered, ordered by increasing PTEN expression level from above down, and plotted in the heat map shown. In the mean centering schema used, red indicates a relatively high level of (phospho)protein expression and green indicates a relatively low level of (phospho)protein expression. The level of PTEN expression is shown in lane 1 and the levels of AKT phosphorylation at Thr308 and Ser473 are shown in lanes 2 and 3, respectively. AKT phosphorylation was expressed as a ratio to total AKT expression for presentation in this figure. Clearly, AKT phosphorylation is strongly inversely correlated with PTEN protein expression. However, there is no clear association between PIK3CA mutation and AKT phosphorylation at either amino acid site. Further, there was no significant difference (P = 0.41) in the frequency of tumors with PIK3CA mutations among those tumors with the highest and lowest quartiles of PTEN expression [17 of 77 (22.1%) and 12 of 77 (15.6%), respectively]. Tumors from one institution batch (i.e., from Clinic Hospital) were used for this analysis.
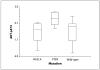
Figure 3
Effect of PTEN and PIK3CA mutations on AKT activation/phosphorylation at Ser473 in 40 breast cancer cell lines. AKT phosphorylation at Ser473 (AKTp473) was significantly higher in _PTEN_-mutant cell lines than in _PIK3CA_-mutant (P = 0.005) or PTEN/PIK3CA wild-type (P = 0.001) cell lines. In contrast, there was no significant difference (P = 0.64) in AKT phosphorylation at Ser473 between PIK3CA-mutant and PTEN/PIK3CA wild-type cell lines. AKTp473 was quantified using RPPA and expressed on the Y axis after logarithmic conversion and mean centering.
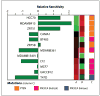
Figure 4
The relative sensitivity of 12 hormone receptor–positive breast cancer cell lines to the PI3K inhibitor LY294002. LY294002 was applied to the panel of hormone receptor–positive breast cancer cell lines and the concentration causing 50% growth inhibition (GI50) was determined and presented as relative sensitivity (−log GI50). The cell lines are presented in order of increasing PTEN protein expression (lane A) as determined using RPPA. PTEN-low (P = 0.0007) and _PTEN_-mutant (P = 0.02) cell lines are significantly more sensitive to growth inhibition by LY294002 than _PIK3CA_-mutant cell lines. Lane B, AKT phosphorylation at Ser473 determined using RPPA [color coded as in lane A (i.e., green, low; black, mean; and red, high AKT phosphorylation)]. Lane C, cell line mutation status.
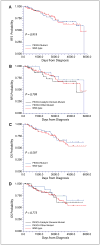
Figure 5
Correlations between PIK3CA mutations and patient survival. No significant differences in RFS (A and B) or OS (C and D) times were found after adjuvant tamoxifen therapy between patients with early-stage PIK3CA wild-type hormone receptor–positive breast tumors and those with _PIK3CA_-mutant breast tumors.
Similar articles
- Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation.
Xing Y, Lin NU, Maurer MA, Chen H, Mahvash A, Sahin A, Akcakanat A, Li Y, Abramson V, Litton J, Chavez-MacGregor M, Valero V, Piha-Paul SA, Hong D, Do KA, Tarco E, Riall D, Eterovic AK, Wulf GM, Cantley LC, Mills GB, Doyle LA, Winer E, Hortobagyi GN, Gonzalez-Angulo AM, Meric-Bernstam F. Xing Y, et al. Breast Cancer Res. 2019 Jul 5;21(1):78. doi: 10.1186/s13058-019-1154-8. Breast Cancer Res. 2019. PMID: 31277699 Free PMC article. Clinical Trial. - Landscape of Phosphatidylinositol-3-Kinase Pathway Alterations Across 19 784 Diverse Solid Tumors.
Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Millis SZ, et al. JAMA Oncol. 2016 Dec 1;2(12):1565-1573. doi: 10.1001/jamaoncol.2016.0891. JAMA Oncol. 2016. PMID: 27388585 - PIK3CA mutations, phosphatase and tensin homolog, human epidermal growth factor receptor 2, and insulin-like growth factor 1 receptor and adjuvant tamoxifen resistance in postmenopausal breast cancer patients.
Beelen K, Opdam M, Severson TM, Koornstra RH, Vincent AD, Wesseling J, Muris JJ, Berns EM, Vermorken JB, van Diest PJ, Linn SC. Beelen K, et al. Breast Cancer Res. 2014 Jan 27;16(1):R13. doi: 10.1186/bcr3606. Breast Cancer Res. 2014. PMID: 24467828 Free PMC article. Clinical Trial. - Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention.
Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro A, Drobot L, Rakus D, Gizak A, Laidler P, Dulińska-Litewka J, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Montalto G, Cervello M, Fitzgerald TL, Demidenko Z, Martelli AM, Cocco L, Steelman LS, McCubrey JA. Davis NM, et al. Oncotarget. 2014 Jul 15;5(13):4603-50. doi: 10.18632/oncotarget.2209. Oncotarget. 2014. PMID: 25051360 Free PMC article. Review. - Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer.
Araki K, Miyoshi Y. Araki K, et al. Breast Cancer. 2018 Jul;25(4):392-401. doi: 10.1007/s12282-017-0812-x. Epub 2017 Oct 31. Breast Cancer. 2018. PMID: 29086897 Review.
Cited by
- Omics analyses of a somatic Trp53R245W/+ breast cancer model identify cooperating driver events activating PI3K/AKT/mTOR signaling.
Yu X, Zhang Y, Xiong S, McDaniel JM, Sun C, Chau GP, Gencel-Augusto J, Chachad D, Morrissey RL, Rao X, Wang J, Lozano G. Yu X, et al. Proc Natl Acad Sci U S A. 2022 Nov 8;119(45):e2210618119. doi: 10.1073/pnas.2210618119. Epub 2022 Nov 2. Proc Natl Acad Sci U S A. 2022. PMID: 36322759 Free PMC article. - Deciphering and Targeting Oncogenic Mutations and Pathways in Breast Cancer.
Santarpia L, Bottai G, Kelly CM, Győrffy B, Székely B, Pusztai L. Santarpia L, et al. Oncologist. 2016 Sep;21(9):1063-78. doi: 10.1634/theoncologist.2015-0369. Epub 2016 Jul 6. Oncologist. 2016. PMID: 27384237 Free PMC article. Review. - Molecular Mechanisms of Trastuzumab Resistance in HER2 Overexpressing Breast Cancer.
Fiszman GL, Jasnis MA. Fiszman GL, et al. Int J Breast Cancer. 2011;2011:352182. doi: 10.4061/2011/352182. Epub 2011 Sep 6. Int J Breast Cancer. 2011. PMID: 22295219 Free PMC article. - The role of SH3GL3 in myeloma cell migration/invasion, stemness and chemo-resistance.
Chen R, Zhao H, Wu D, Zhao C, Zhao W, Zhou X. Chen R, et al. Oncotarget. 2016 Nov 8;7(45):73101-73113. doi: 10.18632/oncotarget.12231. Oncotarget. 2016. PMID: 27683032 Free PMC article. - Predictive, preventive, and personalized medicine in breast cancer: targeting the PI3K pathway.
Tufail M, Hu JJ, Liang J, He CY, Wan WD, Huang YQ, Jiang CH, Wu H, Li N. Tufail M, et al. J Transl Med. 2024 Jan 3;22(1):15. doi: 10.1186/s12967-023-04841-w. J Transl Med. 2024. PMID: 38172946 Free PMC article. Review.
References
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. - PubMed
- Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. - PubMed
- Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30CA16672/CA/NCI NIH HHS/United States
- R01 CA109311/CA/NCI NIH HHS/United States
- P01CA099031/CA/NCI NIH HHS/United States
- U54 CA 112970/CA/NCI NIH HHS/United States
- 1K23CA121994-01/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
- U54 CA112970/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- P01 CA099031/CA/NCI NIH HHS/United States
- P50CA083639/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- K23 CA121994/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- 1R21CA120248-01/CA/NCI NIH HHS/United States
- P50 CA 58207/CA/NCI NIH HHS/United States
- R21 CA120248/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous