IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development - PubMed (original) (raw)
IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development
Kieng B Vang et al. J Immunol. 2008.
Abstract
Common gamma chain (gammac)-receptor dependent cytokines are required for regulatory T cell (Treg) development as gammac(-/-) mice lack Tregs. However, it is unclear which gammac-dependent cytokines are involved in this process. Furthermore, thymic stromal lymphopoietin (TSLP) has also been suggested to play a role in Treg development. In this study, we demonstrate that developing CD4(+)Foxp3(+) Tregs in the thymus express the IL-2Rbeta, IL-4Ralpha, IL-7Ralpha, IL-15Ralpha, and IL-21Ralpha chains, but not the IL9Ralpha or TSLPRalpha chains. Moreover, only IL-2, and to a much lesser degree IL-7 and IL-15, were capable of transducing signals in CD4(+)Foxp3(+) Tregs as determined by monitoring STAT5 phosphorylation. Likewise, IL-2, IL-7, and IL-15, but not TSLP, were capable of inducing the conversion of CD4(+)CD25(+)Foxp3(-) thymic Treg progenitors into CD4(+)Foxp3(+) mature Tregs in vitro. To examine this issue in more detail, we generated IL-2Rbeta(-/-) x IL-7Ralpha(-/-) and IL-2Rbeta(-/-) x IL-4Ralpha(-/-) mice. We found that IL-2Rbeta(-/-) x IL-7Ralpha(-/-) mice were devoid of Tregs thereby recapitulating the phenotype observed in gammac(-/-) mice; in contrast, the phenotype observed in IL-2Rbeta(-/-) x IL-4Ralpha(-/-) mice was comparable to that seen in IL-2Rbeta(-/-) mice. Finally, we observed that Tregs from both IL-2(-/-) and IL-2Rbeta(-/-) mice show elevated expression of IL-7Ralpha and IL-15Ralpha chains. Addition of IL-2 to Tregs from IL-2(-/-) mice led to rapid down-regulation of these receptors. Taken together, our results demonstrate that IL-2 plays the predominant role in Treg development, but that in its absence the IL-7Ralpha and IL-15Ralpha chains are up-regulated and allow for IL-7 and IL-15 to partially compensate for loss of IL-2.
Figures
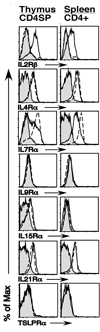
Fig. 1. γ_c_-cytokine-family receptor expression on CD4+Foxp3+ SP thymocytes and CD4+Foxp3+ splenocytes
Thymus and spleen cells from 5–9 week old C57Bl/6 mice were harvested and stained with antibodies to CD4, CD8 and Foxp3, to identify distinct thymic and splenic T cell subsets, as well as with antibodies to IL2Rβ, IL4Rα, IL7Rα, IL9Rα, IL15Rα, IL21Rα and TSLPRα. Shown are flow cytometry histograms of CD4 single positive (SP) thymocytes (left column) and CD4+ splenocytes (right column). Gray filled in histograms represent staining of the corresponding CD4+Foxp3+ population with isotype control antibody. Solid lines and broken lines represent staining of CD4+Foxp3+ and CD4+Foxp3− cells, respectively. A representative example of 4 independent experiments is depicted (n=17).
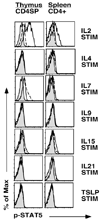
Fig. 2. Cytokine stimulation and Phospho-STAT5 expression on CD4+Foxp3+ thymocytes and splenocytes
Single cell suspensions of thymocytes or splenocytes from Foxp3-GFP mice were serum starved for 30 minutes and then stimulated with IL2, IL4, IL7, IL9, IL15, IL21 or TSLP for 20 minutes. Cells were then stained with antibodies to CD4, CD8, CD25 and phospho-STAT5 as described in the methods section. Shown are histograms of phospho-STAT5 expression in CD4SP thymocytes (left column) and CD4+ splenocytes (right column). Gray filled in histograms represent staining of unstimulated Foxp3-GFP+ cells. Solid lines and broken lines represent staining of stimulated Foxp3-GFP+ and Foxp3-GFP− cells, respectively. A representative example of 2 independent experiments is depicted (n=3 mice). Similar results were obtained when using CD25 to identify Tregs in C57Bl/6 mice (n=13 mice, data not shown).
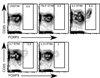
Fig. 3. IL2, IL7 and IL15, but not TSLP, induce the conversion of CD4+CD25+Foxp3− thymic Treg progenitors into CD4+Foxp3+ Tregs
Sorted CD4+CD25+ Foxp3− thymic Treg progenitors from Foxp3-GFP reporter mice were stimulated with 10 U/mL IL2, 5 ng/mL IL7, 100 ng/mL IL15 and 50 ng/mL TSLP in culture overnight. After 24 hours of stimulation, cells were stained with antibodies to CD4 and CD25. Shown are contour plots of Foxp3 versus CD25 for CD4-gated cells (95% of cells were CD4+) after stimulation with medium alone (top left), TSLP (top middle), IL2 (top right), IL7 (bottom left) or IL15 (bottom right). A representative example of 3 independent experiments is depicted.
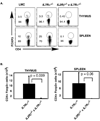
Fig. 4. Treg development in _IL4R_α−/− and _IL2R_β−/− _x IL4R_α−/− mice
Thymus and spleen were harvested from 4–5 week old LMC, _IL4R_α−/−, _IL2R_β−/− and _IL2R_β−/− _x IL4R_α−/− mice. Cells were stained with antibodies to CD4, CD8 and Foxp3 to identify Tregs. Shown are flow cytometry plots of thymus (top panel) or spleen cells (bottom panel) gated on CD4+ T cells. A representative example of 6 independent experiments is depicted.
Fig. 5. Treg development in _IL2R_β−/− and _IL2R_β−/− _x IL7R_α−/− mice
A. Thymus and spleen were harvested from 4–5 week old LMC, _IL7R_α−/−, and _IL2R_β−/− _x IL7R_α−/− mice. Cells were stained with antibodies to CD4, CD8 and Foxp3 to identify Tregs. Shown are flow cytometry plots of thymus (top panel) or spleen cells (bottom panel) gated on CD4+ T cells. A representative example of 6 independent experiments is depicted. B. Shown are bar graphs representing total numbers of CD4+Foxp3+ Tregs in the thymus (left panel) and spleen (right panel). Error bars represent standard error of the mean; n= 6 _IL7R_α−/− and 6 _IL2R_β−/− _x IL7R_α−/− mice; p-values were calculated using two-tailed students t-test.
Fig. 6. Expression of IL2Rβ, IL7Rα and IL15Rα on CD4+Foxp3+ Tregs from _IL2_−/− and _IL2R_β−/− mice compared to wild type littermate controls
Splenocytes were isolated from 4–5 week old LMC, _IL2_−/− and _IL2R_β−/− mice and stained with antibodies for CD4, CD8, and Foxp3 to identify splenic Tregs. Shown are CD4+Foxp3+ gated cells stained for IL2Rβ (left panels), IL7Rα (middle panels) and IL15Rα (right panels). Gray histograms represent staining of CD4+Foxp3+ cells with isotype control antibody. Solid lines represent histograms of CD4+Foxp3+ T cells from _IL2_−/− (top panel) or _IL2R_β−/− (bottom panel) mice; broken lines represent staining of CD4+Foxp3+ Tregs from WT LMC mice. A representative example of 3 independent experiments is depicted (n= 6 _IL2_−/− and 3 _IL2R_β−/− mice).
Fig. 7. Expression of IL7Rα and IL15Rα on CD4+Foxp3+ Tregs in _IL2_−/− mice after ex vivo IL2 stimulation
CD4+ T cells were isolated as described in the methods section and cells were stimulated with 100 units/mL of IL2 for 8 or 24 hours. Cells were then stained for Foxp3, IL7Rα and IL15Rα and analyzed by flow cytometry. Dark unshaded histogram represents IL7Rα and IL15Rα expression after IL2 stimulation for the times indicated. Shaded histograms represent non-stimulated controls. Shown is a representative example of six independent experiments for IL7Rα expression and two independent experiments for IL15Rα expression.
Similar articles
- A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells.
Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. Bayer AL, et al. J Immunol. 2008 Jul 1;181(1):225-34. doi: 10.4049/jimmunol.181.1.225. J Immunol. 2008. PMID: 18566388 Free PMC article. - Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP.
Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Mazzucchelli R, et al. Blood. 2008 Oct 15;112(8):3283-92. doi: 10.1182/blood-2008-02-137414. Epub 2008 Jul 29. Blood. 2008. PMID: 18664628 Free PMC article. - Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma.
Lin SC, Cheng FY, Liu JJ, Ye YL. Lin SC, et al. Int J Mol Sci. 2018 Apr 18;19(4):1231. doi: 10.3390/ijms19041231. Int J Mol Sci. 2018. PMID: 29670037 Free PMC article. Review. - Cytokine Signaling in the Development and Homeostasis of Regulatory T cells.
Toomer KH, Malek TR. Toomer KH, et al. Cold Spring Harb Perspect Biol. 2018 Mar 1;10(3):a028597. doi: 10.1101/cshperspect.a028597. Cold Spring Harb Perspect Biol. 2018. PMID: 28620098 Free PMC article. Review.
Cited by
- In vitro Differentiation of Thymic Treg Cell Progenitors to Mature Thymic Treg Cells.
Owen DL, Farrar MA. Owen DL, et al. Bio Protoc. 2019 Aug 20;9(16):e3335. doi: 10.21769/BioProtoc.3335. eCollection 2019 Aug 20. Bio Protoc. 2019. PMID: 33654840 Free PMC article. - Interleukin-7 influences FOXP3+CD4+ regulatory T cells peripheral homeostasis.
Simonetta F, Gestermann N, Martinet KZ, Boniotto M, Tissières P, Seddon B, Bourgeois C. Simonetta F, et al. PLoS One. 2012;7(5):e36596. doi: 10.1371/journal.pone.0036596. Epub 2012 May 7. PLoS One. 2012. PMID: 22586481 Free PMC article. - Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus.
McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, Grinberg A, Love P, Feigenbaum L, Erman B, Singer A. McCaughtry TM, et al. J Exp Med. 2012 Nov 19;209(12):2263-76. doi: 10.1084/jem.20121505. Epub 2012 Oct 29. J Exp Med. 2012. PMID: 23109710 Free PMC article. - Do Natural T Regulatory Cells become Activated to Antigen Specific T Regulatory Cells in Transplantation and in Autoimmunity?
Hall BM, Tran GT, Verma ND, Plain KM, Robinson CM, Nomura M, Hodgkinson SJ. Hall BM, et al. Front Immunol. 2013 Aug 2;4:208. doi: 10.3389/fimmu.2013.00208. eCollection 2013. Front Immunol. 2013. PMID: 23935597 Free PMC article. - Identification of Cellular Sources of IL-2 Needed for Regulatory T Cell Development and Homeostasis.
Owen DL, Mahmud SA, Vang KB, Kelly RM, Blazar BR, Smith KA, Farrar MA. Owen DL, et al. J Immunol. 2018 Jun 15;200(12):3926-3933. doi: 10.4049/jimmunol.1800097. Epub 2018 May 4. J Immunol. 2018. PMID: 29728511 Free PMC article.
References
- Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. - PubMed
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007;8:351–358. - PubMed
- Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nature immunology. 2005;6:152–162. - PubMed
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI061165-04/AI/NIAID NIH HHS/United States
- AI061165/AI/NIAID NIH HHS/United States
- R01 AI061165-02S1/AI/NIAID NIH HHS/United States
- R01 AI061165-01A1/AI/NIAID NIH HHS/United States
- R01 AI061165-02/AI/NIAID NIH HHS/United States
- R01 AI061165-03/AI/NIAID NIH HHS/United States
- R56 AI061165/AI/NIAID NIH HHS/United States
- R01 AI061165/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous