Interleukin-6 regulates pancreatic alpha-cell mass expansion - PubMed (original) (raw)
. 2008 Sep 2;105(35):13163-8.
doi: 10.1073/pnas.0801059105. Epub 2008 Aug 21.
Jan A Ehses, Eva B Hammar, Leentje Van Lommel, Roel Quintens, Geert Martens, Julie Kerr-Conte, Francois Pattou, Thierry Berney, Daniel Pipeleers, Philippe A Halban, Frans C Schuit, Marc Y Donath
Affiliations
- PMID: 18719127
- PMCID: PMC2529061
- DOI: 10.1073/pnas.0801059105
Interleukin-6 regulates pancreatic alpha-cell mass expansion
Helga Ellingsgaard et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Interleukin-6 (IL-6) is systemically elevated in obesity and is a predictive factor to develop type 2 diabetes. Pancreatic islet pathology in type 2 diabetes is characterized by reduced beta-cell function and mass, an increased proportion of alpha-cells relative to beta-cells, and alpha-cell dysfunction. Here we show that the alpha cell is a primary target of IL-6 actions. Beginning with investigating the tissue-specific expression pattern of the IL-6 receptor (IL-6R) in both mice and rats, we find the highest expression of the IL-6R in the endocrine pancreas, with highest expression on the alpha-cell. The islet IL-6R is functional, and IL-6 acutely regulates both pro-glucagon mRNA and glucagon secretion in mouse and human islets, with no acute effect on insulin secretion. Furthermore, IL-6 stimulates alpha-cell proliferation, prevents apoptosis due to metabolic stress, and regulates alpha-cell mass in vivo. Using IL-6 KO mice fed a high-fat diet, we find that IL-6 is necessary for high-fat diet-induced increased alpha-cell mass, an effect that occurs early in response to diet change. Further, after high-fat diet feeding, IL-6 KO mice without expansion of alpha-cell mass display decreased fasting glucagon levels. However, despite these alpha-cell effects, high-fat feeding of IL-6 KO mice results in increased fed glycemia due to impaired insulin secretion, with unchanged insulin sensitivity and similar body weights. Thus, we conclude that IL-6 is necessary for the expansion of pancreatic alpha-cell mass in response to high-fat diet feeding, and we suggest that this expansion may be needed for functional beta-cell compensation to increased metabolic demand.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
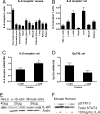
Fig. 1.
IL-6R is expressed in the pancreatic α-cell and is functionally coupled to STAT3 phosphorylation. (A and B) Tissue expression profile of mouse and rat IL-6R mRNA expression determined by Affymetrix gene array (n = 3–5). (C and D) Quantitative RT-PCR on RNA from FACS sorted rat α-cells and β-cells (purity ≈90% as assessed by insulin and glucagon staining respectively) normalized for 18S (n = 3). (E) Western blot analysis of the IL-6R in HeLa cell extracts (+ control), purified rat α-cells and β-cells, and whole mouse islets (representative of n = 3). (F) Western blot of pSTAT3 and total STAT3 in mouse and human islets after 15 min. exposure to 100 ng/ml IL-6 (representative of n = 3). *, P < 0.05.
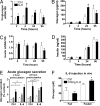
Fig. 2.
Interleukin-6 regulates pro-glucagon mRNA and glucagon secretion with no effect on insulin mRNA and release. (A and C) Pro-glucagon and insulin mRNA in human islets after exposure to 200 ng/ml IL-6 (n = 3–4). (B and D) Glucagon and insulin release in culture medium of human islets after exposure to 200 ng/ml IL-6. (E) Glucagon secretion from human islets during 1 h static incubation in the presence of 20 mM glucose (white bars), 2 mM glucose (hatched bars), and 10 mM Arginine (black bars). Islets were pretreated with 200 ng/ml IL-6 for the indicated times (n = 4). (F) Circulating glucagon levels 2 h after 100 ng bolus IL-6 injection in mice during fed and fasted state (n = 3–5). All secretion experiments were performed on 20 islets per well in triplicate with the number of independent experiments indicated above. *, P < 0.05 vs. respective controls.
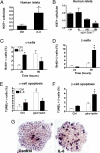
Fig. 3.
Interleukin-6 increases pancreatic α-cell proliferation and prevents α-cell apoptosis in vitro. (A) Ki67-positive human islet-cells per islet after 4 days' treatment in the absence (Ctrl) and presence of 200 ng/ml IL-6 (n = 3–5). (B) Ki67-positive human islet-cells per islet after 4 days treatment with the IL-6R antagonist, Sant7 (200 ng/ml; n = 3). (C and D) Percent BrdU-positive mouse α- and β-cells (glucagon and insulin positive, respectively) of total number of cells. Cells were treated in the absence (Ctrl) and presence of 100 ng/ml IL-6 for 24 and 96 h with BrdU present during the entire experiment (n = 3). (E and F) Percent TUNEL-positive mouse α-cells and β-cells after 12 h treatment with 33.3 mM glucose and 0.5 mM palmitate (gluc + palm), in the absence (Ctrl) and presence of 100 ng/ml IL-6 (n = 3). (G) Representative image of mouse islets on extracellular matrix coated dishes stained for BrdU after 4 days in the absence (control) and presence of 100 ng/ml IL-6, with BrdU present during the entire experiment. *, P < 0.05.
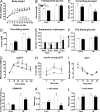
Fig. 4.
Impaired glucose tolerance in IL-6 KO mice after 18 weeks on HF diet. (A) Body weight, (G) ipGTT, (H) glucose-stimulated insulin secretion, and (I) ipITT in WT (solid line, open squares) and IL-6 KO (dashed line, closed circles) mice fed an HF diet for 18 weeks (n = 8 WT, n = 9 IL-6 KO). (B) Fasting blood glucose, (C) fasting plasma glucagon, (D) fasting plasma insulin, (E) glucagon during ipGTT, (F) fed blood glucose, (J) HOMA-IR, (K) a-cell mass, and (L) b-cell mass in WT (white bars) and IL-6 KO (black bars) mice after 18 weeks on HF diet (chow WT n = 5, chow IL-6 KO n = 8, HF WT n = 8, HF IL-6 KO n = 9) *, P < 0.05.
Similar articles
- Role of endogenous IL-6 in the neonatal expansion and functionality of Wistar rat pancreatic alpha cells.
Fernández-Millán E, de Toro-Martín J, Lizárraga-Mollinedo E, Escrivá F, Álvarez C. Fernández-Millán E, et al. Diabetologia. 2013 May;56(5):1098-107. doi: 10.1007/s00125-013-2862-8. Epub 2013 Feb 23. Diabetologia. 2013. PMID: 23435784 - Glycoprotein 130 receptor signaling mediates α-cell dysfunction in a rodent model of type 2 diabetes.
Chow SZ, Speck M, Yoganathan P, Nackiewicz D, Hansen AM, Ladefoged M, Rabe B, Rose-John S, Voshol PJ, Lynn FC, Herrera PL, Müller W, Ellingsgaard H, Ehses JA. Chow SZ, et al. Diabetes. 2014 Sep;63(9):2984-95. doi: 10.2337/db13-1121. Epub 2014 May 8. Diabetes. 2014. PMID: 24812426 - Differentially Expressed MicroRNA-483 Confers Distinct Functions in Pancreatic β- and α-Cells.
Mohan R, Mao Y, Zhang S, Zhang YW, Xu CR, Gradwohl G, Tang X. Mohan R, et al. J Biol Chem. 2015 Aug 7;290(32):19955-66. doi: 10.1074/jbc.M115.650705. Epub 2015 Jun 24. J Biol Chem. 2015. PMID: 26109062 Free PMC article. - Not the second fiddle: α cell development, identity, and function in health and diabetes.
Brooks EP, Sussel L. Brooks EP, et al. J Endocrinol. 2023 Jul 11;258(2):e220297. doi: 10.1530/JOE-22-0297. Print 2023 Aug 1. J Endocrinol. 2023. PMID: 37171828 Free PMC article. Review. - Minireview: Meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion.
Sachdeva MM, Stoffers DA. Sachdeva MM, et al. Mol Endocrinol. 2009 Jun;23(6):747-58. doi: 10.1210/me.2008-0400. Epub 2009 Feb 5. Mol Endocrinol. 2009. PMID: 19196831 Free PMC article. Review.
Cited by
- Nrf2 modulates the benefits of evening exercise in type 2 diabetes.
Fasipe B, Laher I. Fasipe B, et al. Sports Med Health Sci. 2023 Sep 9;5(4):251-258. doi: 10.1016/j.smhs.2023.09.001. eCollection 2023 Dec. Sports Med Health Sci. 2023. PMID: 38314046 Free PMC article. Review. - Rheumatoid Arthritis Treatment Options and Type 2 Diabetes: Unravelling the Association.
Di Muzio C, Cipriani P, Ruscitti P. Di Muzio C, et al. BioDrugs. 2022 Nov;36(6):673-685. doi: 10.1007/s40259-022-00561-7. Epub 2022 Nov 2. BioDrugs. 2022. PMID: 36322327 Free PMC article. - Maternal high-fat diet is associated with altered pancreatic remodelling in mice offspring.
Gregorio BM, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Gregorio BM, et al. Eur J Nutr. 2013 Mar;52(2):759-69. doi: 10.1007/s00394-012-0382-9. Epub 2012 Jun 2. Eur J Nutr. 2013. PMID: 22661265 - Adropin Is Expressed in Pancreatic Islet Cells and Reduces Glucagon Release in Diabetes Mellitus.
Ali II, D'Souza C, Tariq S, Adeghate EA. Ali II, et al. Int J Mol Sci. 2024 Sep 11;25(18):9824. doi: 10.3390/ijms25189824. Int J Mol Sci. 2024. PMID: 39337311 Free PMC article. - Insights on the Role of Putative Muscle-Derived Factors on Pancreatic Beta Cell Function.
Mizgier ML, Fernández-Verdejo R, Cherfan J, Pinget M, Bouzakri K, Galgani JE. Mizgier ML, et al. Front Physiol. 2019 Aug 8;10:1024. doi: 10.3389/fphys.2019.01024. eCollection 2019. Front Physiol. 2019. PMID: 31440170 Free PMC article. Review.
References
- Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–16. - PubMed
- Deng S, et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53:624–632. - PubMed
- Yoon KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. - PubMed
- Donath MY, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–13. - PubMed
- Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials