Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers - PubMed (original) (raw)
Review
Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers
Satoru Kyo et al. Cancer Sci. 2008 Aug.
Abstract
Telomerase activation is a critical step for human carcinogenesis through the maintenance of telomeres, but the activation mechanism during carcinogenesis remains unclear. Transcriptional regulation of the human telomerase reverse transcriptase (hTERT) gene is the major mechanism for cancer-specific activation of telomerase, and a number of factors have been identified to directly or indirectly regulate the hTERT promoter, including cellular transcriptional activators (c-Myc, Sp1, HIF-1, AP2, ER, Ets, etc.) as well as the repressors, most of which comprise tumor suppressor gene products, such as p53, WT1, and Menin. Nevertheless, none of them can clearly account for the cancer specificity of hTERT expression. The chromatin structure via the DNA methylation or modulation of nucleosome histones has recently been suggested to be important for regulation of the hTERT promoter. DNA unmethylation or histone methylation around the transcription start site of the hTERT promoter triggers the recruitment of histone acetyltransferase (HAT) activity, allowing hTERT transcription. These facts prompted us to apply these regulatory mechanisms to cancer diagnostics and therapeutics. Telomerase-specific replicative adenovirus (Telomelysin, OBP-301), in which E1A and E1B genes are driven by the hTERT promoter, has been developed as an oncolytic virus that replicates specifically in cancer cells and causes cell death via viral toxicity. Direct administration of Telomelysin was proved to effectively eradicate solid tumors in vivo, without apparent adverse effects. Clinical trials using Telomelysin for cancer patients with progressive stages are currently ongoing. Furthermore, we incorporated green fluorescent protein gene (GFP) into Telomelysin (TelomeScan, OBP-401). Administration of TelomeScan into the primary tumor enabled the visualization of cancer cells under the cooled charged-coupled device (CCD) camera, not only in primary tumors but also the metastatic foci. This technology can be applied to intraoperative imaging of metastatic lymphnodes. Thus, we found novel tools for cancer diagnostics and therapeutics by utilizing the hTERT promoter.
Figures
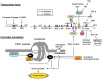
Figure 1
Complex molecular mechanisms of transcriptional regulation of human telomerase reverse transcriptase (hTERT). Representative transcription factors and their upstream factors essential for hTERT regulation are shown in the upper panel. The sites on the promoter are not precisely in scale. +1 indicates the start site of transcription.( 13 ) The proposed model of chromatin remodeling for the regulation of hTERT promoter is shown in the lower panel. Me, methylation of histone; Ac, acetylation of histone.
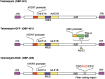
Figure 2
Schematic DNA structures of telomerase‐specific oncolytic viruses. Telomelysin (OBP‐301) has E1A and E1B genes linked with an IRES, driven by the human telomerase reverse transcriptase (hTERT) promoter. A variant of OBP‐301 was constructed that has the green fluorescent protein (GFP) gene at the E3 region driven by CMV promoter (OBP‐401). Another variant (OBP‐405) has a mutant fiber containing the RGD peptide in the HI loop of the fiber knob.
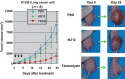
Figure 3
In vivo effect of Telomelysin on tumorigenesis. Lung cancer H1299 cells were inoculated to the flank of nu/n∝ mice. Mice bearing palpable tumors with a diameter of 5–6 mm received intratumoral injection of 107 PFU of Telomelysin or replication‐deficient adenovirus (dL312) or phosphate‐buffered saline (PBS) (mock treatment) on three consecutive days. The macroscopic appearances of H1299 tumors in nu/n∝ mice at 0, 14, and 28 days after the treatment are shown. Note that the tumor growth was severely retarded by the treatment with Telomelysin. A modified version of this figure appeared in our original article.( 106 )
Figure 4
Diagnostic utility of TelomeScan. (a) Visualization of tumor by the injection of TelomeScan. Subcutaneous tumor xenografts by colon cancer (SW620) were directly injected by TelomeScan at concentrations of 8 × 106 PFU. The green fluroescent protein (GFP) fluorescence intensity was monitored for seven consecutive days under the cooled charged‐coupled device (CCD) imaging system. Left panels, macroscopic appearance of subcutaneous tumors; right panels, fluorescence detection. A modified version of this figure appeared in our original article.( 114 ) (b) Selective visualization of lymph node metastasis by TelomeScan in orthotopic xenografts model. The rectums of mice were implanted with mouse rectal cancer HT29 cells. TelomeScan was directly injected into implanted tumor at a concentration of 1 × 108 PFU. At 5 days after the injection, mice were assessed for lymph node metastasis by laparotomy. Three swelled para‐aortic lymph nodes were identified (LN1, LN2, and LN3). Internal imaging with the optical CCD camera showed one of the three nodes with GFP fluorescence (LN3), while the other lymph nodes (LN1, LN2) did not show (arrowheads indicate the position of swelled lymph nodes). Hematoxylin–eosin staining of lymph node sections revealed the apparent metastasis in LN3, while no metastatic sites were identified in LN1 or LN2 (data not shown), indicating that GFP fluorescence by the replication of TelomeScan is a potential biomaker of lymph node metastasis. A modified version of this figure appeared in our original article.( 115 ) (c) Application of TelomeScan to visualization of cervical cancer cells in cytological samples. Uterine cervical scraping cells from patients with cervical cancer were incubated with TelomeScan at 10 MOI for 24 h, and then observed under light microscopy (left panel) or fluorescent microscopy (right panel). Clusters with cellular atypia exhibit GFP fluorescence.
Similar articles
- Diagnostic and therapeutic application of telomerase-specific oncolytic adenoviral agents.
Fujiwara T, Urata Y, Tanaka N. Fujiwara T, et al. Front Biosci. 2008 Jan 1;13:1881-6. doi: 10.2741/2807. Front Biosci. 2008. PMID: 17981675 Review. - Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter.
Fujiwara T, Tanaka N. Fujiwara T, et al. Uirusu. 2008 Jun;58(1):11-8. doi: 10.2222/jsv.58.11. Uirusu. 2008. PMID: 19122384 Review. - [Oncolytic virotherapy for human solid tumors].
Fujiwara T. Fujiwara T. Gan To Kagaku Ryoho. 2009 May;36(5):703-9. Gan To Kagaku Ryoho. 2009. PMID: 19461167 Review. Japanese. - [Theranostic application of telomerase-specific oncolytic adenovirus for human cancer].
Fujiwara T, Tanaka N. Fujiwara T, et al. Nihon Rinsho. 2007 Oct;65(10):1913-22. Nihon Rinsho. 2007. PMID: 17926546 Review. Japanese. - In vivo imaging of human cancer with telomerase-specific replication-selective adenovirus.
Fujiwara T. Fujiwara T. Methods Mol Biol. 2012;872:129-39. doi: 10.1007/978-1-61779-797-2_9. Methods Mol Biol. 2012. PMID: 22700408
Cited by
- hTERT, MYC and TP53 deregulation in gastric preneoplastic lesions.
Silva TC, Leal MF, Calcagno DQ, de Souza CR, Khayat AS, dos Santos NP, Montenegro RC, Rabenhorst SH, Nascimento MQ, Assumpção PP, de Arruda Cardoso Smith M, Burbano RR. Silva TC, et al. BMC Gastroenterol. 2012 Jul 6;12:85. doi: 10.1186/1471-230X-12-85. BMC Gastroenterol. 2012. PMID: 22768805 Free PMC article. - Urinary Phthalates and Leukocyte Telomere Length: An Analysis of NHANES 1999-2002.
Scinicariello F, Feroe AG, Attanasio R. Scinicariello F, et al. EBioMedicine. 2016 Apr;6:96-102. doi: 10.1016/j.ebiom.2016.02.027. Epub 2016 Feb 18. EBioMedicine. 2016. PMID: 27211552 Free PMC article. - RIF1 promotes human epithelial ovarian cancer growth and progression via activating human telomerase reverse transcriptase expression.
Liu YB, Mei Y, Long J, Zhang Y, Hu DL, Zhou HH. Liu YB, et al. J Exp Clin Cancer Res. 2018 Aug 3;37(1):182. doi: 10.1186/s13046-018-0854-8. J Exp Clin Cancer Res. 2018. PMID: 30075819 Free PMC article. - Reptin is required for the transcription of telomerase reverse transcriptase and over-expressed in gastric cancer.
Li W, Zeng J, Li Q, Zhao L, Liu T, Björkholm M, Jia J, Xu D. Li W, et al. Mol Cancer. 2010 May 30;9:132. doi: 10.1186/1476-4598-9-132. Mol Cancer. 2010. PMID: 20509972 Free PMC article. - Correlation of dyskerin expression with active proliferation independent of telomerase.
Alawi F, Lin P, Ziober B, Patel R. Alawi F, et al. Head Neck. 2011 Jul;33(7):1041-51. doi: 10.1002/hed.21579. Epub 2010 Dec 8. Head Neck. 2011. PMID: 21674675 Free PMC article.
References
- Kim NW, Piatyszek MA, Prowse KR et al . Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011–15. - PubMed
- Rudolph KL, Chang S, Lee HW et al . Longevity, stress response, and cancer in aging telomerase‐deficient mice. Cell 1999; 96: 701–12. - PubMed
- Chin L, Artandi SE, Shen Q et al . p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 1999; 97: 527–38. - PubMed
- Artandi SE, DePinho RA. Mice without telomerase: what can they teach us about human cancer? Nat Med 2000; 6: 852–5. - PubMed
- Meyerson M, Counter CM, Eaton EN et al . hEST2, the putative human telomerase catalytic subunit gene, is up‐regulated in tumor cells and during immortalization. Cell 1997; 90: 785–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous