The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis - PubMed (original) (raw)
Review
The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis
Keith D Brown et al. Arthritis Res Ther. 2008.
Abstract
Nuclear factor-kappaB (NF-kappaB) is an inducible transcription factor controlled by two principal signaling cascades, each activated by a set of signal ligands: the classical/canonical NF-kappaB activation pathway and the alternative/noncanonical pathway. The former pathway proceeds via phosphorylation and degradation of inhibitor of NF-kappaB (IkappaB) and leads most commonly to activation of the heterodimer RelA/NF-kappaB1(p50). The latter pathway proceeds via phosphorylation and proteolytic processing of NF-kappaB2 (p100) and leads to activation, most commonly, of the heterodimer RelB/NF-kappaB2 (p52). Both pathways play critical roles at multiple levels of the immune system in both health and disease, including the autoimmune inflammatory response. These roles include cell cycle progression, cell survival, adhesion, and inhibition of apoptosis. NF-kappaB is constitutively activated in many autoimmune diseases, including diabetes type 1, systemic lupus erythematosus, and rheumatoid arthritis (RA). In this review we survey recent developments in the involvement of the classical and alternative pathways of NF-kappaB activation in autoimmunity, focusing particularly on RA. We discuss the involvement of NF-kappaB in self-reactive T and B lymphocyte development, survival and proliferation, and the maintenance of chronic inflammation due to cytokines such as tumor necrosis factor-alpha, IL-1, IL-6, and IL-8. We discuss the roles played by IL-17 and T-helper-17 cells in the inflammatory process; in the activation, maturation, and proliferation of RA fibroblast-like synovial cells; and differentiation and activation of osteoclast bone-resorbing activity. The prospects of therapeutic intervention to block activation of the NF-kappaB signaling pathways in RA are also discussed.
Figures
Figure 1
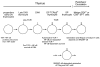
The mammalian families of NF-κB and IκB polypeptides. Conserved domains and their primary functions are indicated. Ankyrins, ankyrin repeat domain (functions by binding and inhibiting RHDs; Bcl-3 and IκBζ are exceptions because they do not function as classical inhibitors of the NF-κB activity); dimeriz., dimerization domain; DNA, DNA binding; NF-κB, nuclear factor-κB; IκB, inhibitor of NF-κB; RHD, Rel homology domain; NLS, nuclear localization sequence; Transactivation, transactivating domain (functions at nuclear target sites).
Figure 2
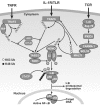
Classical pathway of NF-κB activation via IκB degradation. Ligand engagement of specific membrane receptors triggers K63 polyubiquitination of TRAF2, TRAF6, RIP, MALT1, and NEMO. The TAK kinase complex is recruited through association of the polyubiquitin chains with TAB2 and TAB3. Activated TAK1 may phosphorylate and activate IKKβ, which then phosphorylates IκB bound to cytosolic NF-κB, triggering its β TrCP E3 ubiquitin ligase-mediated K48 polyubiquitination and proteasomal degradation. Free NF-κB then translocates to the nucleus and transactivates target genes. CYLD and A20 are deubiquitinating enzymes that may block NF-κB activation by removal of K63 ubiquitinated chains from activated TRAFs, RIP, and NEMO. A20 may also terminate TNF-α induced NF-κB activation by catalyzing the K48 ubiquitination of RIP, leading to its proteasomal degradation. In addition to promoting survival via NF-κB target genes, the TNF receptor (TNFR1) also stimulates competing apoptotic pathways. T cell (and B cell) antigen receptors (TCR and BCR, respectively [not shown]) may in some contexts enhance apoptotic pathways but usually they contribute to survival (see text). IκB, inhibitor of NF-κB; IKK, IκB kinase; MALT, mucosa-associated lymphoid tissue lymphoma translocation gene; NEMO, NF-κB essential modulator; NF-κB, nuclear factor-κB; RIP, receptor interacting protein; TAB, TAK1-binding protein; TAK, transforming growth factor β-activated kinase; TRAF, TNF receptor-associated factor.
Figure 3
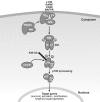
Alternative pathway of NF-κB activation. In unstimulated cells, NIK is destabilized by bound TRAF3. Activation through a subset of receptors of the TNFR superfamily including the BAFFR, CD40, RANK and lymphotoxin-β R leads to the recruitment of TRAF proteins (including TRAF3) to the receptor. TRAF3 is inactivated (possibly by degradation or sequestration) and active NIK is thus released. NIK then phosphorylates and activates IKK; it also recruits NF-κB2/p100 (probably bound to RelB), which is phosphorylated by IKKα. This triggers K48 polyubiquitination of p100 mediated by β TrCP E3 ubiquitin ligase and subsequent proteasomal processing to yield the mature subunit p52. Predominantly RelB/p52 heterodimers are generated, which migrate to the nucleus. The classical pathway is also activated through these receptors with some receptors (BAFFR) activating less strongly than others. Unlike TNFR (Figure 2), BAFFR signaling is associated only with survival functions. BAFFR, B-cell activating factor receptor; IKK, IκB kinase; LT, lymphotoxin; NF-κB, nuclear factor-κB; NIK, NF-κB-inducing kinase; RANK, receptor activator of NF-κB; TNFR, tumor necrosis factor receptor; TRAF, TNF receptor-associated factor.
Figure 4
NF-κB in B-lymphocyte development. A simplified schematic representation of B-lymphocyte development, highlighting some of the contributions of NF-κB at various developmental checkpoints. See text for details. BAFFR, B-cell activating factor receptor; BCR, B-cell receptor; IKK, IκB kinase; NF-κB, nuclear factor-κB; RAG, recombinase-activating gene; T1, transitional 1; T2, transitional 2; TNF, tumor necrosis factor.
Figure 5
NF-κB in T lymphocyte development. A simplified schematic representation of T-lymphocyte development, highlighting some of the contributions of NF-κB at various developmental checkpoints. TReg and NKT cells branch off at some point after TCR expression on thymocytes. See text for details. DP, double-positive stage; DN3/DN4, double-negative stages; IKK, IκB kinase; NF-κB, nuclear factor-κB; NKT, natural killer T cell; SP, single-positive stage; TCR, T-cell receptor; TReg, T-regulatory cell.
Figure 6
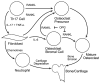
The immune system regulates bone resorption through enhanced osteoclastogenesis. Cells of the adaptive and innate immune systems contribute to regulation of bone turnover through production of cytokines and direct cell-cell interactions. Proinflammatory cytokines such as IL-6, IL-1β, and TNF-α are secreted by macrophages and fibroblasts secrete IL-6. Th17 lymphocytes produce IL-17, IL-6, and TNF-α. In RA these cytokines drive bone erosion by induction of RANKL expression by osteoblast stromal cells. Th17 lymphocytes also secrete RANKL, which binds to RANK receptor on osteoclast precursors triggering osteoclast maturation and activation, thus enhancing bone loss. Osteoprotegerin (OPG) is a soluble decoy receptor that inhibits RANKL binding to RANK thus limiting bone resorption. IL-17 increases RANKL expression and concomitantly decreases OPG expression in osteoblasts, causing enhanced formation of osteoclasts and bone erosion. Neutrophils also contribute to bone and cartilage degradation by secretion of degradative factors. IL, interleukin; RANK, receptor activator of NF-κB; Th, T-helper; TNF, tumor necrosis factor.
Similar articles
- The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development.
Dejardin E. Dejardin E. Biochem Pharmacol. 2006 Oct 30;72(9):1161-79. doi: 10.1016/j.bcp.2006.08.007. Epub 2006 Sep 12. Biochem Pharmacol. 2006. PMID: 16970925 Review. - Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde?
Noort AR, Tak PP, Tas SW. Noort AR, et al. Arthritis Res Ther. 2015 Jan 28;17(1):15. doi: 10.1186/s13075-015-0527-3. Arthritis Res Ther. 2015. PMID: 25774937 Free PMC article. Review. - TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation.
Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S. Saitoh T, et al. J Biol Chem. 2003 Sep 19;278(38):36005-12. doi: 10.1074/jbc.M304266200. Epub 2003 Jul 1. J Biol Chem. 2003. PMID: 12840022 - Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology.
Beinke S, Ley SC. Beinke S, et al. Biochem J. 2004 Sep 1;382(Pt 2):393-409. doi: 10.1042/BJ20040544. Biochem J. 2004. PMID: 15214841 Free PMC article. Review. - Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis.
Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, Kim HY. Kim KW, et al. Arthritis Res Ther. 2005;7(1):R139-48. doi: 10.1186/ar1470. Epub 2004 Nov 29. Arthritis Res Ther. 2005. PMID: 15642134 Free PMC article.
Cited by
- The Crosstalk of Pathways Involved in Immune Response Maybe the Shared Molecular Basis of Rheumatoid Arthritis and Type 2 Diabetes.
Niu X, Lu C, Xiao C, Ge N, Jiang M, Li L, Bian Y, Xu G, Bian Z, Zhang G, Lu A. Niu X, et al. PLoS One. 2015 Aug 7;10(8):e0134990. doi: 10.1371/journal.pone.0134990. eCollection 2015. PLoS One. 2015. PMID: 26252209 Free PMC article. - Controlling the fate of NIK: a central stage in noncanonical NF-kappaB signaling.
Sun SC. Sun SC. Sci Signal. 2010 May 25;3(123):pe18. doi: 10.1126/scisignal.3123pe18. Sci Signal. 2010. PMID: 20501935 Free PMC article. - Melittin acupoint injection in attenuating bone erosion in collagen-induced arthritis mice via inhibition of the RANKL/NF-κB signaling pathway.
Liu F, Chen F, Yang L, Qiu F, Zhong G, Gao S, Xi W, Lai M, He Q, Chen Y, Chen W, Zhang J, Yang L. Liu F, et al. Quant Imaging Med Surg. 2023 Sep 1;13(9):5996-6013. doi: 10.21037/qims-23-254. Epub 2023 Jul 20. Quant Imaging Med Surg. 2023. PMID: 37711782 Free PMC article. - Effects of progesterone on hippocampal ultrastructure and expression of inflammatory mediators in neonatal rats with hypoxic-ischemic brain injury.
Li X, Zhang J, Zhu X, Hou R, Li X, Dong X, Wang X, Lu C. Li X, et al. Exp Ther Med. 2014 May;7(5):1311-1316. doi: 10.3892/etm.2014.1589. Epub 2014 Feb 27. Exp Ther Med. 2014. PMID: 24940430 Free PMC article. - Novel application of multi-stimuli network inference to synovial fibroblasts of rheumatoid arthritis patients.
Kupfer P, Huber R, Weber M, Vlaic S, Häupl T, Koczan D, Guthke R, Kinne RW. Kupfer P, et al. BMC Med Genomics. 2014 Jul 3;7:40. doi: 10.1186/1755-8794-7-40. BMC Med Genomics. 2014. PMID: 24989895 Free PMC article.
References
- Brown K, Claudio E, Siebenlist U. New developments in NF-kappa B. In: Smolen JS, Lipsky PE, editor. Contemporary Targeted Therapies in Rheumatology. London: Informa; 2007. pp. 285–296.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials