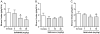A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats - PubMed (original) (raw)
A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats
Carsten K Nielsen et al. Biol Psychiatry. 2008.
Abstract
Background: Naltrexone, a compound with high affinity for the mu opioid receptor (MOP-R) reduces alcohol consumption. SoRI-9409 is a derivative of naltrexone that has highest affinity at delta opioid receptors (DOP-Rs). We have investigated the effects of SoRI-9409 on ethanol consumption to determine the consequences of altering the naltrexone compound to a form with increased efficacy at DOP-Rs.
Methods: Effects of the opioid receptor antagonists, SoRI-9409 (0-30 mg/kg, IP), naltrexone (0-30 mg/kg, IP), or naltrindole (0-10 mg/kg, IP) on ethanol consumption was measured in high- and low-ethanol-consuming rats with two different drinking paradigms. SoRI-9409-, naltrexone-, and naltrindole-mediated inhibition of DOP-R-stimulated [(35)S]GTP gamma S binding was measured in brain membranes prepared from high-ethanol-consuming rats. The effects of SoRI-9409 on morphine-mediated analgesia, conditioned place preference, and anxiety were also examined.
Results: In high- but not low-ethanol-consuming animals, SoRI-9409 is threefold more effective and selective at reducing ethanol consumption when compared with naltrexone or naltrindole for up to 24 hours. SoRI-9409 administered daily for 28 days continuously reduced ethanol consumption, and when the administration of SoRI-9409 was terminated, the amount of ethanol consumed remained lower compared with vehicle-treated animals. Furthermore, SoRI-9409 inhibits DOP-R-stimulated [(35)S]GTP gamma S binding in brain membranes of high-ethanol-consuming rats.
Conclusions: SoRI-9409 causes selective and long-lasting reductions of ethanol consumption. This suggests that compounds that have high affinity for DOP-Rs such as SoRI-9409 might be promising candidates for development as a novel therapeutic for the treatment of alcoholism.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures
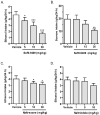
Figure 1
SoRI-9409 selectively reduces ethanol intake in high-ethanol–consuming rats. (A) SoRI-9409 (5, 15, and 30 mg/kg IP, n = 12) significantly and dose-dependently decreases ethanol consumption in rats, using the intermittent access to 20% ethanol drinking paradigm. (B) SoRI-9409 (5 and 15 mg/kg IP, n = 10) does not significantly affect sucrose consumption, using the two-bottle-choice 5% sucrose drinking paradigm. (C) Naltrexone (15 and 30 mg/kg IP, n = 12) significantly decreases ethanol consumption and (D). Naltrindole (1, 5, and 10 mg/kg IP, n = 12) does not significantly decrease ethanol consumption, using the intermittent access to 20% ethanol drinking paradigm. The values are expressed as mean ± SEM ethanol or sucrose consumed (g/kg/24 hours) (repeated measures analysis of variance followed by Newman-Keuls post hoc test *p < .05; **p < .01; ***p < .001; compared with vehicle).
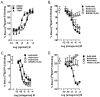
Figure 2
SoRI-9409 reduces ethanol intake, using the continuous access 10% ethanol two-bottle-choice drinking paradigm. (A) SoRI-9409 (5, 15, and 30 mg/kg IP) significantly and dose-dependently decreases ethanol consumption in rats after 24 hours. (B) Naltrexone (5, 15, and 30 mg/kg IP) does not significantly decrease ethanol consumption. (C) Naltrindole (1, 5, and 10 mg/kg IP) significantly decreases ethanol consumption. The values are expressed as mean ± SEM ethanol consumed (g/kg/24 hours) (repeated measures analysis of variance followed by Newman-Keuls post hoc test). *p < .05; compared with vehicle, n = 12.
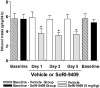
Figure 3
Multiple dosing of SoRI-9409 reduces ethanol intake in high-ethanol–consuming rats. Daily administration of SoRI-9409 (5 mg/kg IP) for 5 days (three drinking sessions) to long-term drinking rats significantly reduced ethanol intake in rats after 24 hours of ethanol access. Daily administration of vehicle to long-term drinking rats for 5 days did not affect ethanol intake (p > .05). When daily administrations of SoRI-9409 (or vehicle) were ceased, rats returned to baseline levels of ethanol intake. The values are expressed as mean ± SEM ethanol consumed (g/kg/24 hours) (Student t test). *p < .05; compared with baseline levels of ethanol intake, n = 8.
Figure 4
SoRI-9409 inhibits δ opioid receptor (DOP-R)-stimulated [35S]GTPγS binding in the striatum of long-term heavy drinking rats. (A) The DOP-R ligands SNC80, DPDPE, and TAN67 significantly stimulate [35S]GTPγS binding in the striatum of long-term heavy drinking rats. (B) SoRI-9409 and naltrindole but not naltrexone inhibit SNC80-stimulated [35S]GTPγS DOP-R striatal binding. (C) SoRI-9409, naltrindole, and naltrexone inhibit DPDPE-stimulated [35S]GTPγS striatal binding. (D) SoRI-9409 but not naltrindole or naltrexone inhibits TAN67-stimulated [35S]GTPγS DOP-R striatal binding. The values are expressed as mean ± SEM percentage increase in basal [35S]GTPγS binding.
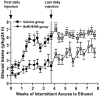
Figure 5
Long-term treatment with SoRI-9409 reduces escalation of ethanol intake and induces a long-lasting reduction in ethanol intake when the drug is no longer present. Rats were administered either SoRI-9409 (5 mg/kg IP n = 16, closed circles) or vehicle (n = 15, closed squares) on each of 28 consecutive days, 30 min before the drinking session. SoRI-9409 significantly reduced ethanol consumption after 24 hours of ethanol access at each drinking session. The administration of either SoRI-9409 (open circles) or vehicle (open squares) was terminated after 28 days (12 ethanol exposures). Ethanol consumption in the SoRI-9409-treated but not the vehicle-treated group remained lower for a further 28 days. The values are expressed as mean ± SEM ethanol consumed (g/kg/24 hours) (Student t test). *p < .05; **p < .01; ***p < .001.
Similar articles
- The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking.
Nielsen CK, Simms JA, Bito-Onon JJ, Li R, Ananthan S, Bartlett SE. Nielsen CK, et al. Addict Biol. 2012 Mar;17(2):224-34. doi: 10.1111/j.1369-1600.2010.00295.x. Epub 2011 Feb 11. Addict Biol. 2012. PMID: 21309957 Free PMC article. - SoRI 9409, a non-peptide opioid mu receptor agonist/delta receptor antagonist, fails to stimulate [35S]-GTP-gamma-S binding at cloned opioid receptors.
Xu H, Lu YF, Rice KC, Ananthan S, Rothman RB. Xu H, et al. Brain Res Bull. 2001 Jul 1;55(4):507-11. doi: 10.1016/s0361-9230(01)00550-0. Brain Res Bull. 2001. PMID: 11543951 - δ-opioid receptor function in the dorsal striatum plays a role in high levels of ethanol consumption in rats.
Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, Santos N, Bartlett SE. Nielsen CK, et al. J Neurosci. 2012 Mar 28;32(13):4540-52. doi: 10.1523/JNEUROSCI.5345-11.2012. J Neurosci. 2012. PMID: 22457501 Free PMC article. - Opiates and alcohol self-administration in animals.
Ulm RR, Volpicelli JR, Volpicelli LA. Ulm RR, et al. J Clin Psychiatry. 1995;56 Suppl 7:5-14. J Clin Psychiatry. 1995. PMID: 7673105 Review. - Endogenous opioid systems and alcohol addiction.
Herz A. Herz A. Psychopharmacology (Berl). 1997 Jan;129(2):99-111. doi: 10.1007/s002130050169. Psychopharmacology (Berl). 1997. PMID: 9040115 Review.
Cited by
- Levetiracetam results in increased and decreased alcohol drinking with different access procedures in C57BL/6J mice.
Fish EW, Agoglia AE, Krouse MC, Muller RG, Robinson JE, Malanga CJ. Fish EW, et al. Behav Pharmacol. 2014 Feb;25(1):61-70. doi: 10.1097/FBP.0000000000000019. Behav Pharmacol. 2014. PMID: 24322822 Free PMC article. - Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading.
Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Simms JA, et al. Neuropsychopharmacology. 2010 Jun;35(7):1453-63. doi: 10.1038/npp.2010.15. Epub 2010 Mar 3. Neuropsychopharmacology. 2010. PMID: 20200505 Free PMC article. - Leveraging Neural Networks in Preclinical Alcohol Research.
Smith LC, Kimbrough A. Smith LC, et al. Brain Sci. 2020 Aug 21;10(9):578. doi: 10.3390/brainsci10090578. Brain Sci. 2020. PMID: 32825739 Free PMC article. Review. - Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats.
Li J, Zou Y, Ye JH. Li J, et al. Brain Res Bull. 2011 Nov 25;86(5-6):428-34. doi: 10.1016/j.brainresbull.2011.08.013. Epub 2011 Aug 27. Brain Res Bull. 2011. PMID: 21893169 Free PMC article. - Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats.
Henderson-Redmond A, Czachowski C. Henderson-Redmond A, et al. Psychopharmacology (Berl). 2014 Nov;231(22):4309-21. doi: 10.1007/s00213-014-3571-9. Epub 2014 Apr 26. Psychopharmacology (Berl). 2014. PMID: 24770627 Free PMC article.
References
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. - PubMed
- Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–688. - PubMed
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. - PubMed
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adreno-cortical axis. Psychopharmacology (Berl) 2002;160:19–29. - PubMed
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials