Effects of structure of Rho GTPase-activating protein DLC-1 on cell morphology and migration - PubMed (original) (raw)
Effects of structure of Rho GTPase-activating protein DLC-1 on cell morphology and migration
Tai Young Kim et al. J Biol Chem. 2008.
Abstract
DLC-1 encodes a Rho GTPase-activating protein (RhoGAP) and negative regulator of specific Rho family proteins (RhoA-C and Cdc42). DLC-1 is a multi-domain protein, with the RhoGAP catalytic domain flanked by an amino-terminal sterile alpha motif (SAM) and a carboxyl-terminal START domain. The roles of these domains in the regulation of DLC-1 function remain to be determined. We undertook a structure-function analysis involving truncation and missense mutants of DLC-1. We determined that the amino-terminal SAM domain functions as an autoinhibitory domain of intrinsic RhoGAP activity. Additionally, we determined that the SAM and START domains are dispensable for DLC-1 association with focal adhesions. We then characterized several mutants for their ability to regulate cell migration and identified constitutively activated and dominant negative mutants of DLC-1. We report that DLC-1 activation profoundly alters cell morphology, enhances protrusive activity, and can increase the velocity but reduce directionality of cell migration. Conversely, the expression of the amino-terminal domain of DLC-1 acts as a dominant negative and profoundly inhibits cell migration by displacing endogenous DLC-1 from focal adhesions.
Figures
FIGURE 1.
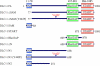
Truncation and missense mutants of DLC-1 for DLC-1 structure-function analyses. A schematic representation of the DLC-1 truncation constructs is shown. The full-length and various fragments of DLC-1 cDNA were subcloned into the EGFP-tagged expression vector, pEGFP-N1, resulting in the expression of carboxyl-terminal GFP-tagged fusion proteins.
FIGURE 2.
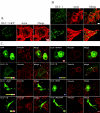
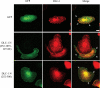
Morphology of cells transfected with DLC-1 constructs and DLC-1 subcellular localization. A, DLC-1 negative MDA-MB-468 cells grown on glass coverslips were transiently transfected with EGFP-tagged full-length DLC-1, stained with rhodamine-phalloidin, and imaged with a confocal fluorescence microscope to observe subcellular localization and the effects on cell morphology. Scale bar, 10 μm. B, DLC-1 positive MDA-MB-231 cells were fixed and stained with anti-DLC-1 antibody followed by anti-mouse secondary antibodies conjugated to Alexa Fluor 488 and rhodamine-phalloidin (upper panel). A higher magnification of the analysis is shown in the lower panels. Scale bar, 20 μm.C, the various EGFP-tagged DLC-1 constructs indicated were transiently transfected into MDA-MB-468 cells, and the cells were stained with an anti-vinculin antibody followed by a Alexa Fluor 594 labeled secondary antibody. Selected overlap regions of vinculin and DLC-1 are indicated with_white arrows. Scale bar_, 10 μm.
FIGURE 3.
SAM domain deleted DLC-1 shows enhanced catalytic activity for RhoA. A, bacterially expressed full-length, SAM domain deleted (amino acids 77-1091), and RhoGAP domain fragment (amino acids 609-878) of DLC-1 were purified for analysis of in vitro GAP activity. Purified GST-RhoA fusion proteins were preloaded with GTP, and GTP hydrolysis was monitored by incubation with a phosphate-binding protein that undergoes a major increase in fluorescence upon binding inorganic phosphate. B, GTP hydrolysis activities of DLC-1 constructs. C, GTP loading of RhoA in cells was monitored by a Rhotekin pull-down assay as described under “Experimental Procedures.”
FIGURE 4.
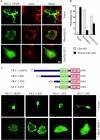
Effects of active RhoA and Cdc42 on the morphology of cells expressing DLC-1. A, HEK293 cells were cotransfected with pEGFP-DLC-1 ΔSAM and vector alone (pAX142) or constructs expressing active forms of RhoA(Q63L) or Cdc42(12V) and replated on fibronectin-coated coverslips (10 μg/ml) for 1 h. The overall cell shapes as well as the localization of DLC-1 and of actin filaments were imaged by confocal fluorescence microscopy.Scale bar, 10 μm. Unusual, multi-branched shapes were frequently seen in cells transfected with pEGFP-DLC-1 ΔSAM as illustrated in the_top row. B_, the bar graph shows the fraction of cells displaying long, branched, neurite-like projections (black bars) and cells displaying flat morphology similar to untransfected HEK293 cells (gray bars) in the experiment described in A. The means ± S.D. of ∼100 cells analyzed in three independent assays are shown. C, HEK293 cells were cotransfected with various amino-terminal truncations of pEGFP-DLC-1 and with RhoA(Q63L) or control vector. The overall cell shapes as well as the localization of DLC-1 were imaged as above.
FIGURE 5.
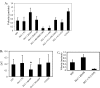
Migration of cells transfected with various truncations or mutations of DLC-1. For migration experiments HEK293 cells transfected with various truncation constructs or mutants of DLC-1 were plated on fibronectin-coated (10 μg/ml) 35-mm glass-bottomed culture dishes for 30 min to 1 h. Digital images were taken every 5 min for a total of 6 h/experiment. A, the migration speeds of the cells (15-25 cells/condition) were determined by dividing the total length of migration path by the total time elapsed.B, directional persistence (D/T) was determined by dividing the net displacement (D) by total length of the migration path (T). A and B illustrate results for HEK293 cells transfected with various pEGFP-DLC-1 constructs or treated with the Rho kinase inhibitor Y27632. In B only the DLC-1 ΔSAM transfected cells (star) displayed a statistically significant difference from EGFP transfected controls. This observation was repeated in several independent experiments. C, MDA-MB-231 cells were transfected with various DLC-1 truncation constructs and migration speeds of the cells (15-20 cells/condition) were determined as described above.
FIGURE 6.
Active DLC-1 reduces persistence of cell movement. The images were taken at the indicated time (min) and illustrate the rapid changes in directionality (lack of persistence) in cells transiently transfected with pEGFP-DLC-1 ΔSAM as compared with control cells transfected with the pEGFP empty vector. The arrows indicate the direction of cell movement. The images presented are representative of multiple observations.
FIGURE 7.
The amino terminus of DLC-1 displaces endogenous DLC-1 from focal contacts. MDA-MB-231 cells were transiently transfected with pEGFP, pEGFP-DLC-1 ΔN (252-508), or pEGFP-DLC-1 ΔN (252-1091, R718E). These proteins retain the tensin-binding sequence that leads to focal adhesion localization but do not have Rho GAP activity, nor are they recognized by the antibody that binds full-length DLC-1. Transfected cells were then were replated on fibronectin-coated coverslips (10 μg/ml) for 4-5 h and stained with anti-DLC-1 monoclonal antibodies (to visualize endogenous DLC-1) followed by anti-mouse secondary antibodies conjugated to Alexa Fluor 594 and imaged with a confocal fluorescence microscope. The arrows indicate the focal adhesions. Scale bar, 10 μm. The images presented are representative of multiple observations.
Similar articles
- DLC-1 suppresses non-small cell lung cancer growth and invasion by RhoGAP-dependent and independent mechanisms.
Healy KD, Hodgson L, Kim TY, Shutes A, Maddileti S, Juliano RL, Hahn KM, Harden TK, Bang YJ, Der CJ. Healy KD, et al. Mol Carcinog. 2008 May;47(5):326-37. doi: 10.1002/mc.20389. Mol Carcinog. 2008. PMID: 17932950 Free PMC article. - Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility.
Kim TY, Vigil D, Der CJ, Juliano RL. Kim TY, et al. Cancer Metastasis Rev. 2009 Jun;28(1-2):77-83. doi: 10.1007/s10555-008-9167-2. Cancer Metastasis Rev. 2009. PMID: 19221866 Free PMC article. Review. - Mutations in the focal adhesion targeting region of deleted in liver cancer-1 attenuate their expression and function.
Liao YC, Shih YP, Lo SH. Liao YC, et al. Cancer Res. 2008 Oct 1;68(19):7718-22. doi: 10.1158/0008-5472.CAN-08-2042. Cancer Res. 2008. PMID: 18829524 Free PMC article. - Identifying Cancer-Relevant Mutations in the DLC START Domain Using Evolutionary and Structure-Function Analyses.
Holub AS, Bouley RA, Petreaca RC, Husbands AY. Holub AS, et al. Int J Mol Sci. 2020 Oct 31;21(21):8175. doi: 10.3390/ijms21218175. Int J Mol Sci. 2020. PMID: 33142932 Free PMC article. - DLC-1:a Rho GTPase-activating protein and tumour suppressor.
Durkin ME, Yuan BZ, Zhou X, Zimonjic DB, Lowy DR, Thorgeirsson SS, Popescu NC. Durkin ME, et al. J Cell Mol Med. 2007 Sep-Oct;11(5):1185-207. doi: 10.1111/j.1582-4934.2007.00098.x. J Cell Mol Med. 2007. PMID: 17979893 Free PMC article. Review.
Cited by
- Deleted in liver cancer protein family in human malignancies (Review).
Lukasik D, Wilczek E, Wasiutynski A, Gornicka B. Lukasik D, et al. Oncol Lett. 2011 Sep 1;2(5):763-768. doi: 10.3892/ol.2011.345. Epub 2011 Jul 5. Oncol Lett. 2011. PMID: 22866123 Free PMC article. - Frequent Downregulation and Promoter Hypermethylation of DLC1: Relationship with Clinical Outcome in Gallbladder Cancer.
Singh D, Bharti A, Biswas D, Tewari M, Kar AG, Ansari MA, Singh S, Narayan G. Singh D, et al. J Gastrointest Cancer. 2022 Jun;53(2):237-244. doi: 10.1007/s12029-020-00560-3. Epub 2021 Jan 8. J Gastrointest Cancer. 2022. PMID: 33417200 - Differential regulation of the activity of deleted in liver cancer 1 (DLC1) by tensins controls cell migration and transformation.
Cao X, Voss C, Zhao B, Kaneko T, Li SS. Cao X, et al. Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1455-60. doi: 10.1073/pnas.1114368109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22307599 Free PMC article. - SH3 domain regulation of RhoGAP activity: Crosstalk between p120RasGAP and DLC1 RhoGAP.
Chau JE, Vish KJ, Boggon TJ, Stiegler AL. Chau JE, et al. Nat Commun. 2022 Aug 15;13(1):4788. doi: 10.1038/s41467-022-32541-4. Nat Commun. 2022. PMID: 35970859 Free PMC article. - Mechanical regulation of talin through binding and history-dependent unfolding.
Dahal N, Sharma S, Phan B, Eis A, Popa I. Dahal N, et al. Sci Adv. 2022 Jul 15;8(28):eabl7719. doi: 10.1126/sciadv.abl7719. Epub 2022 Jul 15. Sci Adv. 2022. PMID: 35857491 Free PMC article.
References
- Jaffe, A., and Hall, A. (2005) Annu. Rev. Cell Dev. Biol. 21 247-269 - PubMed
- Raftopoulou, M., and Hall, A. (2004) Dev. Biol. 265 23-32 - PubMed
- Rossman, K. L., Der, C. J., and Sondek, J. (2005) Nat. Rev. Mol. Cell Biol. 6 167-180 - PubMed
- Moon, S. Y., and Zheng, Y. (2003) Trends Cell Biol. 13 13-22 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous