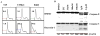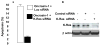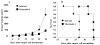Identification of a small molecule with synthetic lethality for K-ras and protein kinase C iota - PubMed (original) (raw)
Identification of a small molecule with synthetic lethality for K-ras and protein kinase C iota
Wei Guo et al. Cancer Res. 2008.
Abstract
K-Ras mutations are frequently found in various cancers and are associated with resistance to treatment or poor prognosis. Similarly, poor outcomes have recently been observed in cancer patients with overexpression of protein kinase C iota (PKCiota), an atypical protein kinase C that is activated by oncogenic Ras protein and is required for K-Ras-induced transformation and colonic carcinogenesis in vivo. Thus far, there is no effective agent for treatment of cancers with K-Ras mutations or PKCiota overexpression. By synthetic lethality screening, we identified a small compound (designated oncrasin-1) that effectively kills various human lung cancer cells with K-Ras mutations at low or submicromolar concentrations. The cytotoxic effects correlated with apoptosis induction, as was evidenced by increase of apoptotic cells and activation of caspase-3 and caspase-8 upon the treatment of oncrasin-1 in sensitive cells. Treatment with oncrasin-1 also led to abnormal aggregation of PKCiota in the nucleus of sensitive cells but not in resistant cells. Furthermore, oncrasin-1-induced apoptosis was blocked by siRNA of K-Ras or PKCiota, suggesting that oncrasin-1 is targeted to a novel K-Ras/PKCiota pathway. The in vivo administration of oncrasin-1 suppressed the growth of K-ras mutant human lung tumor xenografts by >70% and prolonged the survival of nude mice bearing these tumors, without causing detectable toxicity. Our results indicate that oncrasin-1 or its active analogues could be a novel class of anticancer agents, which effectively kill K-Ras mutant cancer cells.
Figures
Figure 1
Library screening. (A) Chemical structures of oncrasin-1. (B) Dose effect of oncrasin-1 in T29, T29Ht1, and T29Kt1 cells. The cells were treated with various concentrations (ranging from 0.1 μM to 33 μM) of oncrasin-1. Cell viability was determined 3 days after treatment by SRB assays. Control cells were treated with solvent (DMSO), and their value was set as 1. (C) Dose-Response in human lung cancer cell lines. Lung cancer cells with various oncogenic Ras gene status were treated with oncrasin-1 at various concentrations. Cell viability was determined as in B. The values shown are the means + SD of two assays done in quadruplicate. Control cells were treated with solvent (DMSO), whose value was set as 1. (D) The status of Ras gene and p53 gene mutations, and IC50 of oncrasin-1 in lung cancer cell lines tested in C. *Based on published data and the Cancer Genome Project Database (
http://www.sanger.ac.uk/genetics/CGP/
). Wt, wild type; UN, unknown; HBEC, human bronchial epithelial cells.
Figure 2
Apoptosis induction by oncrasin-1. (A) T29, T29Kt1, and H460 cells were treated with 10 μM (for T29 or T29Kt1) or 1 μM (for H460) oncrasin-1 and then harvested 12 hours later. Apoptosis was detected by flow cytometiric analysis. The number in each panel indicates percentage of apoptotic cells. (B) Western blot analysis. H460 cells were treated with various concentrations of oncrasin-1 as indicated or with 30 μM indole-3-carbinol (I3C), an inactive analogue, for 24 hours. Activation of caspases 3 and 8 was detected by Western blot analysis. β-actin was used as the loading control.
Figure 3
K-Ras knockdown inhibited oncrasin-mediated apoptosis. (A) H460 human lung cancer cells were treated with either control (luciferase [Luc]) siRNA or K-Ras siRNA and then treated with DMSO or 1 μM oncrasin-1 for 12 hours. The values represent differences of percentage of apoptotic cells between oncrasin-1 + siRNA and DMSO + siRNA groups. The values shown are the means + SD of two experiments. (B) Western blot analysis of siRNA-mediated K-Ras knockdown in H460 cells. cells treated with phosphate-buffered saline, K-Ras siRNA, or control siRNA were harvested for testing K-Ras proteins by Western blot analysis. β-Actin was used as the loading control.
Figure 4
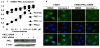
Oncrasin-induced aggregation of PKCι T29, T29Kt1, and H460 cells were treated with DMSO or 10 μM (for T29 or T29Kt1) or 1 μM (for H460) oncrasin-1 for 12 hours, and then immunofluorescent staining was performed to test for intracellular localization of PKCι. Nuclear PKCι aggregated into large foci after oncrasin-1 treatment in sensitive T29Kt1 and H460 cells but not in the resistant T29 cells.
Figure 5
Effects of PKCι on oncrasin-1-induced cytotoxicity. (A) Susceptibility to oncrasin-1 after PKCι Knockdown. Six plasmids encoding PKCι specific shRNA were divided into two groups (PKCι i-1 and PKCι i-2) and were used in the experiment. PKCζ shRNA plasmids (a mixture of 4) were used as a control. Cell viability was determined as described in Figure 1. Western blot analysis of PKCι in T29Kt1 cells after plasmid transfection and a brief selection was also shown. (B) Knockdown of PKCι abolished abnormal nuclear PKCι aggregation. The experiment was performed as described in Figure 4.
Figure 6
Antitumor activity in vivo. (A) Tumor growth. Mice with subcutaneous tumors derived from H460 cells were treated with oncrasin-1 or solvent. Tumor volumes were monitored over time after the treatments. The values are the means ± SD of data from 5 mice per group. The mean tumor volume in the mice treated with oncrasin-1 differed significantly from that of the solvent-treated mice (p <.05). (B) Survival curve. The mean survival times in mice treated with solvent and oncrasin-1 were 24 and 32 days, respectively.
Similar articles
- Interruption of RNA processing machinery by a small compound, 1-[(4-chlorophenyl)methyl]-1H-indole-3-carboxaldehyde (oncrasin-1).
Guo W, Wu S, Wang L, Wang RY, Wei X, Liu J, Fang B. Guo W, et al. Mol Cancer Ther. 2009 Feb;8(2):441-8. doi: 10.1158/1535-7163.MCT-08-0839. Epub 2009 Feb 10. Mol Cancer Ther. 2009. PMID: 19208825 Free PMC article. - Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis.
Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, Fields AP. Regala RP, et al. Cancer Res. 2009 Oct 1;69(19):7603-11. doi: 10.1158/0008-5472.CAN-09-2066. Epub 2009 Sep 8. Cancer Res. 2009. PMID: 19738040 Free PMC article. - Antitumor activity of a novel oncrasin analogue is mediated by JNK activation and STAT3 inhibition.
Guo W, Wu S, Wang L, Wei X, Liu X, Wang J, Lu Z, Hollingshead M, Fang B. Guo W, et al. PLoS One. 2011;6(12):e28487. doi: 10.1371/journal.pone.0028487. Epub 2011 Dec 12. PLoS One. 2011. PMID: 22174819 Free PMC article. - Across the universe of K-RAS mutations in non-small-cell-lung cancer.
Piva S, Ganzinelli M, Garassino MC, Caiola E, Farina G, Broggini M, Marabese M. Piva S, et al. Curr Pharm Des. 2014;20(24):3933-43. doi: 10.2174/13816128113196660761. Curr Pharm Des. 2014. PMID: 24138715 Review. - The RAS-RAL axis in cancer: evidence for mutation-specific selectivity in non-small cell lung cancer.
Guin S, Theodorescu D. Guin S, et al. Acta Pharmacol Sin. 2015 Mar;36(3):291-7. doi: 10.1038/aps.2014.129. Epub 2015 Jan 5. Acta Pharmacol Sin. 2015. PMID: 25557115 Free PMC article. Review.
Cited by
- Aphanin, a triterpenoid from Amoora rohituka inhibits K-Ras mutant activity and STAT3 in pancreatic carcinoma cells.
Rabi T, Catapano CV. Rabi T, et al. Tumour Biol. 2016 Sep;37(9):12455-12464. doi: 10.1007/s13277-016-5102-2. Epub 2016 Jun 22. Tumour Biol. 2016. PMID: 27333990 - Antitumor activity of a novel STAT3 inhibitor and redox modulator in non-small cell lung cancer cells.
Liu X, Guo W, Wu S, Wang L, Wang J, Dai B, Kim ES, Heymach JV, Wang M, Girard L, Minna J, Roth JA, Swisher SG, Fang B. Liu X, et al. Biochem Pharmacol. 2012 May 15;83(10):1456-64. doi: 10.1016/j.bcp.2012.02.010. Epub 2012 Feb 22. Biochem Pharmacol. 2012. PMID: 22387047 Free PMC article. - Development of synthetic lethality anticancer therapeutics.
Fang B. Fang B. J Med Chem. 2014 Oct 9;57(19):7859-73. doi: 10.1021/jm500415t. Epub 2014 Jun 13. J Med Chem. 2014. PMID: 24893124 Free PMC article. Review. - Defeat mutant KRAS with synthetic lethality.
Pang X, Liu M. Pang X, et al. Small GTPases. 2017 Oct 2;8(4):212-219. doi: 10.1080/21541248.2016.1213783. Epub 2016 Jul 27. Small GTPases. 2017. PMID: 27463838 Free PMC article. Review. - Synthetic Lethality in Lung Cancer-From the Perspective of Cancer Genomics.
Shimomura I, Yamamoto Y, Ochiya T. Shimomura I, et al. Medicines (Basel). 2019 Mar 12;6(1):38. doi: 10.3390/medicines6010038. Medicines (Basel). 2019. PMID: 30871030 Free PMC article. Review.
References
- Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–7. - PubMed
- Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. - PubMed
- Bernhard EJ, Stanbridge EJ, Gupta S, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597–6600. - PubMed
- Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–38. - PubMed
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Rev Mol Cell Biol. 2005;6:167–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA 098582/CA/NCI NIH HHS/United States
- R01 CA092487/CA/NCI NIH HHS/United States
- CA 70907/CA/NCI NIH HHS/United States
- R01 CA098582/CA/NCI NIH HHS/United States
- R01 CA092487-04A2/CA/NCI NIH HHS/United States
- R01 CA092487-05/CA/NCI NIH HHS/United States
- CA 16672/CA/NCI NIH HHS/United States
- R01 CA 092487/CA/NCI NIH HHS/United States
- P50 CA070907/CA/NCI NIH HHS/United States
- R01 CA098582-04/CA/NCI NIH HHS/United States
- P50 CA070907-04/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous